Autor : De Vito, Eduardo L.1,2, Arce Santiago C.1, Monteiro Sergio G.1
1Medical Research Institute Alfredo Lanari, Faculty of Medicine, University of Buenos Aires, Buenos Aires, Argentina. 2Centro del Parque, Respiratory Care Department, Buenos Aires, Argentina.
https://doi.org/10.56538/ramr.YPZI6030
Correspondencia : Eduardo Luis De Vito, E-mail: eldevito@gmail.com
ABSTRACT
When confronted with a physical
stimulus, not everyone feels the same way; each one expresses a sensation with
different words; not all of us describe respiratory sensations in the same way;
and, why not say it, not all professionals understand what the patient tells
them. The psychophysics of dyspnea (quantitative relationships between a respiÂratory
stimulus and a sensation) and descriptors for shortness of breath (the dyspnea
language) can help break down communication barriers between patients, family,
and health care personnel. General data support a cortical-limbic network for
the perception of dyspnea. The insular cortex is widely agreed to be an
essential central component of neural circuitry, while the anterior cingulate
cortex and dorsolateral prefrontal cortex are thought to modulate the magnitude
of dyspnea perception and its relief. Dyspnea has been confirmed in
neuroimaging studies as a central nervous system phenomenon, with both sensory
and affective dimensions. It has been firmly established that dyspnea is a
complex mind-body experience consisting of different sensations that can only
be perceived by the individual. The accompanying feelings of distress, fear, and
anxiety are driven by affective components, and it is the brain, not the lungs,
the one that genÂerates these phenomena.
Key words: Dyspnea, Physiology, Physiopathology, Psychophysics, Descriptors
RESUMEN
No
todos sentimos lo mismo ante un estÃmulo fÃsico, cada uno expresa una sensaciÃģn
con diferentes palabras, no todos describimos de igual forma las sensaciones
respiÂratorias y, por quÃĐ no decirlo, no todos los profesionales entienden lo
que el paciente les relata. La psicofÃsica de la disnea (las relaciones
cuantitativas entre un estÃmulo respiratorio y una sensaciÃģn), los descriptores
para referirse a la falta de aire (el lenguaje de la disnea) pueden ayudar a
romper las barreras comunicacionales entre pacientes, familia y personal de salud. Los datos generales apoyan una red cortical-lÃmbica
para la percepciÃģn de la disnea. Hay acuerdo en que la corteza insular es un
elemento central esencial para el circuito neuronal, mientras que la corteza cingulada anterior y la corteza prefrontal
dorsolateral se cree que modulan la magnitud de la
percepciÃģn de disnea y su alivio. La disnea como un fenÃģmeno del sistema
nervioso central y con dimensiones tanto sensoriales como afectivas, esto ha
sido confirmado en estudios de neuroimÃĄgenes. Se ha
establecido firmemente que la disnea es una experiencia compleja de la mente y
el cuerpo, que comprende diferentes sensaciones que solo pueden ser percibidas
por el individuo. Los componentes afectivos impulsan los sentimientos
acompaÃąantes de angustia, miedo y ansiedad, y es el cerebro, no los pulmones,
el que genera estos fenÃģmenos.
Palabras
clave: Disnea,
FisiologÃa, FisiopatologÃa, PsicofÃsica, Descriptores
Received: 12/03/2022
Accepted: 01/16/2024
PSYCHOPHYSICAL LAWS IN GENERAL
No historical account of dyspnea
would be complete without mentioning the role of psychophysics. A detailed
analysis of this topic is beyond the scope of this article. There are excellent
publications by Mahler1,2 that can be consulted. Psychophysical laws are
a set of mathematical expressions that attempt to determine quantitative
relationships between the stimulus or input parameters and the sensation or
output parameters (perception responses).
The study of non-respiratory
sensations dates back to the mid-19th century. The German phyÂsician and
physicist Hermann von Helmholtz coined the term âpsychophysicsâ and established
a precise and non-linear relationship between the magnitude of physical stimuli
and the perceived intensity. Helmholtz paved the way for the develÂopment of
âpsychophysical lawsâ. The essential authors of the 19th century are Weber and
FechÂner, while Stevens and Borg represent the second half of the 20th century
and are credited with the application of psychophysical measures to respiraÂtory
sensations.
In 1846, Weber reported that the
just noticeÂable difference in intensity between two stimuli is a constant
fraction of the intensity of the first stimulus:
Just Noticeable = delta stimulus (it is a constant)
Difference Stimulus
Meaning, the greater the base
stimulus (e.g., a sound), the larger the change in stimulus magniÂtude must be
to detect it (this does not hold true for extreme stimuli).1
In the late 1950s, Stevens was
able to study responses for various sensory modes (light, sound, taste, smell,
touch, muscle force, movement).2-4 He expressed
the relationship between the intensity of the stimulus and the magnitude of the
sensation with his psychophysical law (or power law):
S = c Ek
Where S is the magnitude of the sensation; c is an arbitrary constant; E is
the intensity of the stimulus, and k is the exponent that depends on the
sensory modality and environmental condiÂtions. The exponent k is very
relevant as it provides information on how the stimulus is sensorily
processed.
â When k = 1 (visual appreciation
of the length of a straight line), the psychological magnitude corresponds
directly to changes in the stimulus;
â When it is >1 (electric
shock, temperature), small changes in the magnitude of the stimulus expand
across a wide range of psychological magnitude,
â and
when it is <1 (light, sound), wide ranges of stimulus magnitude are judged
as small in terms of psychological magnitude.
PSYCHOPHYSICS OF DYSPNEA
Bakers and Tenney
in 1970 were the first to apply Stevensâ Law to respiratory variables.5 In fact,
within the respiratory system, the sensory experiÂence
is more complex and is studied in terms of relationships between inspiratory
pressure and resulting sensation. Not all respiratory stimuli have the same
exponent.
The Weberâs law would have
important impliÂcations for the study of patients with abnormal respiratory
mechanics, in whom airway resistance and/or lung elastance
are often increased. Studies from that time established that:
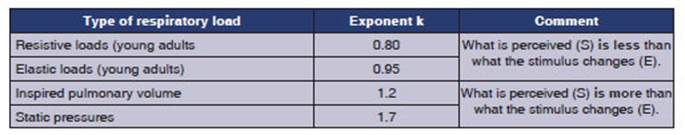
â Normal subjects over 60 years
old perceive less elastic and resistive loads compared to normal subjects under 30 years (they have lower expoÂnent k values).
â Patients with COPD (chronic
obstructive pulmoÂnary disease) perceive less resistive loads (they have lower
exponent k values).
â Normal subjects and asthmatics
perceive equalÂly (they have the same exponent k), except in the group with
near-fatal asthma (they have lower exponent k values, perceiving less).
Psychophysical laws and the Borg Scale
The result of applying these laws
was the concepÂtion in 1982 of the well-known Borg Scale and other similar
scales.1-4 The Borg
Scale (initially created for the perception of dyspnea during exÂercise) was
able to reconcile an absolute sensory magnitude (0 to 10) with quantitative
semantics (mild, moderate, severe, etc.). With some modificaÂtions, it is
widely used today to quantify dyspnea and muscle discomfort during physical
activity. Furthermore, between 1981 and 1989, it was posÂsible to reach two
conclusions of interest:5,6
â The intensity of discomfort is
proportional to the deviation from the spontaneous ventilatory
patÂtern. This highlighted the exquisite mechanisms operating to
minimize dyspnea in physiological and pathological situations.
â Temporal adaptation, according
to which senÂsory magnitude declines in accordance with a simple exponential
function over time (and depending on the magnitude of the respiratory stimulus
or load) helped to explain why certain patients can be remarkably asymptomatic
with high-intensity stimuli and/or chronic overstimuÂlation.
However, it is worth mentioning
that with a better understanding of the multidimensional nature of dyspnea, new
precise scales have been developed that evaluate the sensory and affective
components of the sensation, and their use should be part of routine care for
certain patients.7
The language of
dyspnea. Descriptors
Our ability to conceptualize and
communicate an idea depends on our success in bringing the idea to life through
language, and in turn, the physician must be able to decode that language.
When a physician encounters a
patient who reports chest pain, they usually ask a series of questions about
the intensity and quality of the painful sensation. On a scale from 0-10, what
score would you give to your pain? What characÂteristics does it have? Does it
vary with breathing or coughing? Does it radiate to another part of the body? Traditional
texts used by medical school students do not discuss the qualitative aspects of
dyspnea, perhaps because it is often considered a single sensation.
The concept of dyspnea quality
has been present since the times of Comroe,
Campbell, and Guz, but it wasnât until 1990 that the
task of developing a language for dyspnea began, allowing patients and
physicians to communicate about inherent respiratory discomfort. In fact, the
current defiÂnition of dyspnea includes qualitatively different sensations.8
The attempts to associate certain
conditions or diseases with qualitatively specific sensations did not yield the
desired results. It is not possible to reasonably assert that a particular type
of sensaÂtion corresponds to a disease to the extent that it can be
diagnostically oriented. There are multiple physiological mechanisms underlying
dyspnea in different stages of the disease, as well as multiple sensations that
can coexist within a particular patient.
The language of dyspnea is based
on how it is communicated, and therefore, the spectrum of descriptors is broad.9-11 Some of them
are used more frequently than others, and while they allow us to understand the
distress they generate and the impact produced by that distress (including a
sensation of death), they cannot be considered by any
means a clinical guide to direct the causes of dyspnea. Descriptors have been
developed by Simon,9-11 but we
do not have a Spanish validation of them.
Attention to the use of
verbal descriptors of dyspnea can help the clinician avoid underestimatÂing the
severity of airflow limitation when it is not possible to take objective
measurements of the lung function. However, there is certain overlap that
cannot be ignored, even though the trends appear to be consistent:
â The descriptor âincreased
work of breathingâ is associated with COPD, moderate to severe asthma,
myopathy, and pulmonary fibrosis.
â Patients with COPD
and dynamic hyperinÂflation sometimes complain of a sensation of âunsatisfactory/incomplete/short
and quick breathsâ or a feeling of ânot being able to take deep
breaths.â
â A âfeeling of
rapid and shallow breathingâ may correspond to interstitial lung disease or
decreased compliance of the chest wall.
â Heart failure is
also associated with a sensation of âsuffocation/breathlessness.â
â A sensation of âheavy
breathingâ is typical of deconditioning.
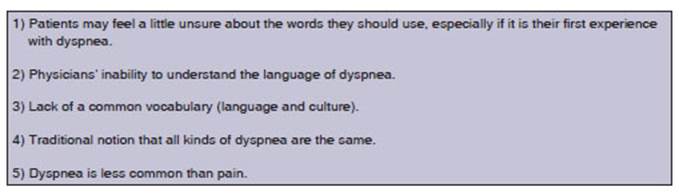
There are multiple
communication barriers to understanding the language of dyspnea (Table 2). By
developing dyspnea questionnaires, physicians and their patients are more
likely to communiÂcate accurately about respiratory symptoms and mechanisms.
It is important to remember
that an individuÂalâs language, gender, ethnic origin, and culture can
influence the wording they use to describe dyspnea.11
CENTRAL PROCESSING OF DYSPNEA
Cortical substrate for the perception of dyspnea
â Neurophysiological
studies through evoked potential testing.12-14
â Imaging studies:
positron emission tomography (PET), functional magnetic resonance imaging
(fMRI) with blood oxygenation level dependent technique (BOLD).15-17
Neurophysiological studies - phrenic afferents
The first study to
establish a neurophysiological link between phrenic afferents and the somatosenÂsory
cortex was conducted by Frankstein.12 Until
the 1980s, there was a deeply rooted belief that reflexes mediated by afferents
in the diaphragm were irrelevant or absent. This conception began to change
when it was discovered that approxiÂmately 30-45% of the fibers of the phrenic
nerve are sensory afferents. It is undeniable that higher centers are
interested in the type of activity and the contractile state of the diaphragm.
Phrenic afferents contribute to the somatosensation
of the diaphragm, conscious perception of breathing, and responses to
respiratory load.18 Figure 1 shows these projections.
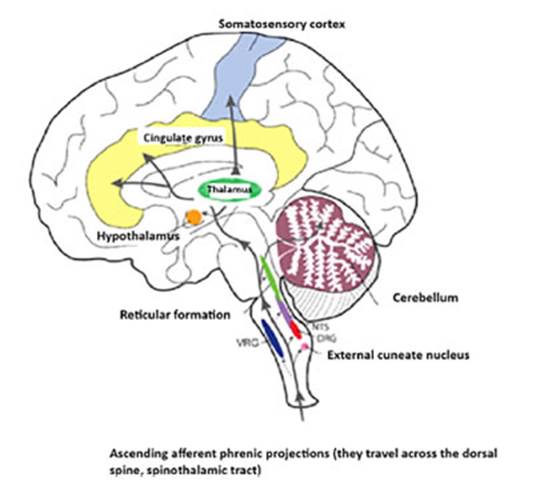
The fact that phrenic afferents
also project to the limbic system in humans suggests a possible link between
diaphragmatic sensory afferents and the emotional state: Cortical evoked
responses to brief inspiratory occlusions are strongly modulated by affective
state in humans. Phrenic afferents may also be involved in shoulder or neck
pain. This response likely reflects the activation of group III-IV phrenic
afferents that converge with the spiÂnothalamic tract
in the high cervical spinal cord.21
In summary, animal data confirm that diaÂphragmatic sensory afferents activate
neurons in the somatosensory cortex, and human data are entirely consistent
with these observations. In addition to modulating respiratory patterns, inÂformation
transmitted through phrenic afferents contributes to diaphragmatic somatosensation and conscious perception of breathing.
There is still much to learn
about the potential role of phrenic afferents in the activation or moduÂlation
of respiratory neuroplasticity, particularly in the context of rehabilitation
following neurological injury and/or neuromuscular disease.
Functional imaging studies
While in the early 1990s it was
postulated that the rostral projections of respiratory motor neurons from the
brainstem to the midbrain and thalamus could represent the central corollary
discharge pathway to the sensory cortex,15
until 1994 the cortical region processing information related to dyspnea
remained unidentified.
A PET study on the activation of
the respiratory motor command during CO2 breathing provided the
first indication that limbic areas could be involved in the perception of
dyspnea.22 It was posÂsible to identify neuronal activation in the
upper brainstem, midbrain, hypothalamus, thalamus, hippocampus and parahippocampus, fusiform gyrus,
cingulate area, insula (considered the fifth cerebral lobe), frontal cortex, temporo-occipital cortex, and parietal cortex. This
activation was considered relevant in sensory and motor respiÂratory responses
to hypercapnia in awake indiÂviduals.22,23
Hypercapnia per se produces dyspnea,
regardless of the increase in ventilation induced by CO2 (Figure 2).24
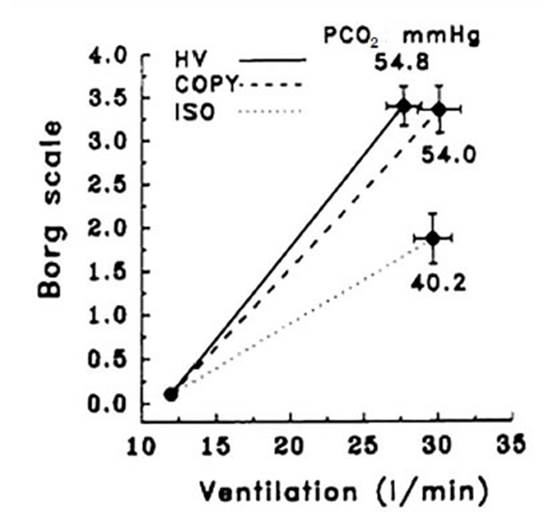
For equal levels of
hyperventilation (HV), durÂing hypercapnia (54.8
mmHg), the sensation of dyspnea was greater than during isocapnia
(ISO, 40.2 mmHg). In this group of healthy volunteers, CO2 induced
dyspnea independently of the conÂcomitant increase in ventilation.24
Consistent with these findings, Karley et al found that limbic and paralimbic
areas activated by CO2 were located in the anterior insula,
operculum, cerebellum, amygdala, thalamus, and basal ganÂglia. Some frontoparietal elements related to atÂtention were also
activated.25 Respiratory variables represented in these areas
included hypercapnia, variations in tidal volume (TV),
inspiratory and expiratory resistive loads, and variations in tidal volume
under mechanical ventilatory assistance.16
Brain imaging is unable to distinguish between structures involved in affective
and discriminative processing and motor behavioral responses.26,27
In summary, studies
suggested that the insula is essential for the perception of dyspnea, although
current data suggest that the insula acts in concert with a notably extensive
and complex neuronal network.
Sensory and affective components
The pivotal studies from 1995 to
2000 have proÂvided compelling evidence that sensory intenÂsity and unpleasantness
of pain are different dimensions. They even appear to be dependent on
separate neural pathways.28-30 Consistent with these findings, a multidimensional
model of dysÂpnea has been proposed with two components: a) sensory (i.e.,
intensity and quality) and b) affecÂtive (evaluative, unpleasant).15,16,30 Davenport and Reep
described the two main suggested pathways for processing respiratory sensation
in the sensory cortex.31
1) It is believed that sensory
aspects (intenÂsity and quality) predominantly originate in afferents
located in the respiratory muscles (phrenic afferents and others), are transmitÂted
to the brainstem, and are projected to the ventral area of the thalamus, from
where thalaÂmocortical projections ascend to the
primary somatosensory cortex (Brodmann areas 3, 1,
and 2) and secondary cortex (Brodmann areas 5 and 7).16,26,30
2) Affective components
(evaluative, unÂpleasant) appear to go through
another pathway. Information, mainly vagal afferents from the lungs and
airways, is projected to the brainstem. Brainstem projections ascend to the
amygdala and the dorsomedial area of the thalamus and
beyond the insula and cinÂgulate cortex. These structures are part of the
limbic system, which forms the inner border of the cortex and contains rich
interconnections between the cerebral cortex, the thalamus, and the brainstem.
The limbic system is also considered important for reward, fear, hunger,
thirst, and sexual arousal. The thalamus and hippocampus are believed to be
critical neural areas for respiratory sensory input to the ceÂrebral cortex.16,30
How does the insula give rise to the perception of dyspnea?
Although there is growing
evidence suggesting that the insular cortex acts as a center for interoÂception and plays a fundamental role in the awareÂness
of subjective feelings rather than simply a role in processing the perception
of unpleasantness, it is worth asking how the insula gives rise to the
perception of dyspnea.32
It has been suggested that
increased corollary discharges from the medullary motor command of the
brainstem to the respiratory muscles can activate the insula, presumably even without
peÂripheral afferent feedback from respiratory mechaÂnoreceptors.
Furthermore, although it is unclear whether pain and dyspnea are processed by
the same cortical structures or simply by neighboring cortical structures, it
is evident that the insular cortex plays an important role in the perception of
both sensations.
Lessons from specific clinical situations
As mentioned, by the end of the
first decade of the 21st century, the multidimensionality similar to the
perception of pain and dyspnea began to be suggested, and includes sensory
components (i.e., intensity and quality) and affective components. This
approach has clinical implications.30-32
1) High sensitivity seems to be
favorable because it allows for early detection of deteriorating lung function
and rapid relief with medication.
2) A moderate degree of
asthma-related anxiety is adaptive because it may be associated with a better
perception of bronchoconstriction.
3) On the other hand, the absence
of anxiety can lead to indifference and neglect of symptoms.33
4) An exaggerated perception of
dyspnea, which can lead to excessive use of medical resources, may imply an
excessive response in the affective dimension.
5) The affective dimension of
dyspnea (displeasure, emotional response) appears not to strictly deÂpend on
the intensity of dyspnea.
Davenport et al used
respiratory-evoked potenÂtial methodology in a group of asthmatic children with
a history of near-fatal asthma.13 They
found an absence of an evoked component in 6/11 chilÂdren after respiratory
occlusion (i.e., the sensory signal of dyspnea was not activating the somatoÂsensory
cortex). These data suggest the presence of a specific deficit in nearly fatal
asthma in the cortical processing of respiratory load informaÂtion. It is not
yet possible to determine whether patients with decreased perception of dyspnea
have a specific deficit in the affective rather than the sensory aspects of
their perceptual processing.
CONCLUSIONS
A differentiation between the
sensory and affecÂtive components of dyspnea may be particularly important in
improving the accuracy of symptom perception. Neuroimaging studies have shed
light on the brain networks involved in the perception of the sensory and
affective components of dyspnea. It remains to be determined whether this can
contribute to the development of more effective therapeutic strategies for
patients with dyspnea.
Neurobiology of dyspnea, endogenous and exogenous opioids
In 1985, Santiago and Edelman
postulated that endogenous opioids could be elaborated as a protective
mechanism to relieve difficulty breathing.34 In
2009, OâDonnell proposed a neurobiological model (Figure 3) involving the
respiratory and nervous systems that has alÂlowed us to improve our
understanding of the perception of dyspnea.35
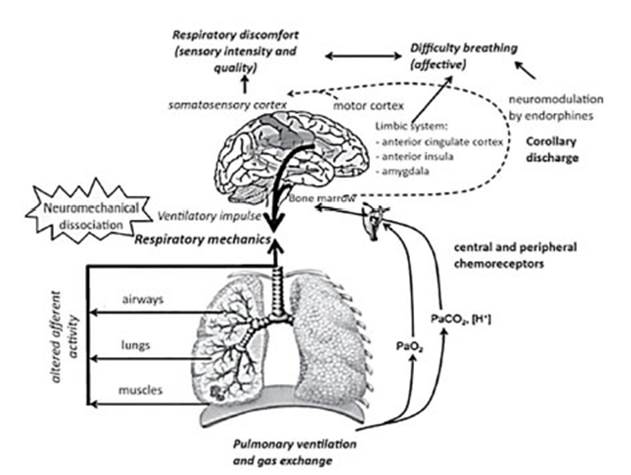
The respiratory system is
modulated by excitÂatory and inhibitory neuropeptides acting from sensory
neurons to central networks. Endogenous opioids are inhibitory neuropeptides
that affect reÂspiratory rate and nociception. When administerÂing 10 mg of
naloxone IV to block opioid receptor signaling, COPD patients reported higher
scores of difficulty breathing compared to normal saline administration, both
during exercise and with reÂsistive load breathing. These results suggest that
endogenous opioids modify dyspnea by acting on the CNS. Opioids modulate
dyspnea perception by decreasing the central respiratory drive (and asÂsociated
corollary discharge), altering the central perception, and/or reducing anxiety.7,35
The fear of an overdose and the
development of respiratory depression has historically
limited the use of opioids to alleviate dyspnea in the clinical practice.
However, recent statements from two major global pulmonology societies27,36 recommend that oral and parenteral opioids be used
for the relief of refractory dyspnea. Refractory dyspnea is defined as âdyspnea
that persists at rest or with minimal activity and is distressing despite
optimal treatment of advanced lung or heart disease.â In addition to proper
titration, communication beÂtween physicians, patients, and family members is
essential when using opioids for palliative and end-of-life care.36
The American Thoracic Society
proposed in 2012 that dyspnea be considered in three conÂstructs:
sensory, affective, and the impact or burden of symptoms. (Table 3).36
The intensity (sensory) and distress (affective) in
response to a specific stimulus have already been discussed. The impact of
dyspnea on an individualâs daily activities can be considered either during
patient care or in a clinical trial. Most of the instruments that are currently
being used to quantify dyspnea in clinical trials are relatively recent, dating
back to only 30 years approximately (Table 3).

The neurophysiological model
provides a conÂceptual framework to enhance our understanding of the mechanisms
contributing to the perception of dyspnea. The opioid system plays a
significant role in relieving dyspnea. Both endogenous opioids (β-endorphins) and exogenous opioids (morphine analogs) modulate dyspnea.
Interventions that stimulate the release of endogenous opioids require further
research to alleviate dyspnea.7
CONCLUSIONS
Comroeâs vision on dyspnea as a central nervous system phenomenon, with both
sensory and afÂfective dimensions was premonitory and has now been confirmed in
neuroimaging studies. Yesterday and today, dyspnea is a primary experience asÂsociated
with behaviors aimed at counteracting a threat to survival. It has been firmly
established that dyspnea is a complex mind-body experience that consists of
different sensations that can only be perceived by the individual. Affective
compoÂnents drive the accompanying feelings of distress, fear, and anxiety, and
it is the brain, not the lungs, the one that generates these phenomena.7,36-38
REFERENCES
1. Mahler D. Dyspnea. Medicine & Science in Sports & ExÂercise. 1991;23:1322. http://dx.doi.org/10.1249/00005768-199111000-00027
2. Donald A, Mahler DO. Dyspnea, Mechanisms, MeasureÂment and
Management. Donald A. Mahler DO, editor. CRC Press;
2005. (2nd Edition).
3. Stevens SS. On
the psychophysical law. Psychol Rev. 1957;64:153-81. https://doi.org/10.1037/h0046162
4. Borg GA. Psychophysical bases
of perceived exertion. Med Sci
Sports Exerc. 1982;14:377-81.
https://doi.org/10.1249/00005768-198205000-00012
5. Bakers JH, Tenney
SM. The perception of some sensations associated with breathing. Respir Physiol. 1970;10:85-92.
https://doi.org/10.1016/0034-5687(70)90029-0
6. Roussos C, Macklem
PT. The Thorax. Roussos C, Macklem PT, editors. Vol. 42. Marcel Decker; 1986.
https://doi.org/10.1016/S0003-4975(10)61851-6
7.
Mahler DA, OâDonnell DE. Recent advances in
dysÂpnea. Chest. 2015;147:232-41.
https://doi.org/10.1378/ chest.14-0800
8. Dyspnea. Mechanisms,
assessment, and management: a consensus statement. American
Thoracic Society. Am J Respir Crit Care Med. 1999;159:321-40.
https://doi.org/10.1164/ajrccm.159.1.ats898
9. Simon PM, Schwartzstein
RM, Weiss JW, et al. DistinÂguishable sensations of breathlessness induced in
normal volunteers. Am Rev Respir Dis. 1989;140:1021-7. https://doi.org/10.1164/ajrccm/140.4.1021
10. Simon PM, Schwartzstein
RM, Weiss JW, Fencl V, TeghtÂsoonian
M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness
of breath. Am Rev Respir Dis. 1990;142:1009-14.
https://doi.org/10.1164/ajrccm/142.5.1009
11. Hardie
GE, Brown JK, Gold WM. Bronchial hyperresponÂsiveness,
word descriptors, and ethnicity: women with mild asthma. J Asthma.
2012;49:36-44.
https://doi.org/10.3109/02770903.2011.637839
12.
Frankstein SI, Smolin LN, Sergeeva ZN, Sergeeva TI. Cortical representation of the phrenic nerve. Exp Neurol. 1979;63:447-
9. https://doi.org/10.1016/0014-4886(79)90139-0
13. Davenport PW, Cruz M, Stecenko AA, Kifle Y.
Respiratory-related evoked potentials in children with life-threatening asthma.
Am J Respir Crit Care Med.
2000;161:1830-5. https://doi.org/10.1164/ajrccm.161.6.9903077
14.
Nicot FJ, Renault FR, Flores-Guevara RR. Respiratory-related evoked potentials in children with neuromuscular
diseases. Clinical Neurophysiology. 2012;123:e26-7. http://dx.doi.org/10.1016/j.clinph.2011.11.117
15. Nishino T. Dyspnoea: underlying mechanisms and treatment. Br J Anaesth. 2011;106:463-74.
https://doi.org/10.1093/bja/aer040
16. Herigstad
M, Hayen A, Wiech K, Pattinson KTS. Dyspnoea and the brain. Respir Med. 2011;105:809-17. https://doi.org/10.1016/j.rmed.2010.12.022
17. Marlow LL, Faull OK, Finnegan
SL, Pattinson KTS. Breathlessness and the brain: the
role of expectation. Curr Opin Support Palliat
Care. 2019;13:200-10.
https://doi.org/10.1097/SPC.0000000000000441
18. Frazier DT, Revelette WR. Role of phrenic nerve
afferents in the control of breathing. J Appl
Physiol. 1991;70:491-6.
https://doi.org/10.1152/jappl.1991.70.2.491
19. Bałkowiec
A, Kukuła K, Szulczyk
P. Functional classificaÂtion of afferent phrenic nerve fibres
and diaphragmatic receptors in cats. J Physiol. 1995;483:759-68.
https://doi.org/10.1113/jphysiol.1995.sp020620
20. Jammes
Y, Balzamo E. Changes in afferent and efferent
phrenic activities with electrically induced diaphragÂmatic fatigue. J Appl Physiol. 1992;73:894-902.
https://doi.org/10.1152/jappl.1992.73.3.894
21. Nair J, Streeter KA, Turner
SMF, Sunshine MD, Bolser DC,
Fox EJ, et al. Anatomy and physiology of phrenic afferent neurons. J Neurophy K, H siol. 2017 Dec 1;118(6):2975-90. https://doi.org/10.1152/jn.00484.2017
22. Corfield DR, Fink GR, Ramsay SC, Murphyarty
HR, Watson JD, et al. Evidence for limbic system activation during CO2-
stimulated breathing in man. J Physiol. 1995;488:77-84.
https://doi.org/10.1113/jphysiol.1995.sp020947
23. Straus C, Zelter M, Derenne JP, Pidoux B, Willer JC, Similowski T. Putative projection of phrenic afferents to
the limbic cortex in humans studied with cerebral-evoked potentials. J Appl Physiol. 1997;82:480-90.
https://doi.org/10.1152/jappl.1997.82.2.480
24. De Vito EL, Roncoroni AJ, Berizzo EE, Pessolano F. Effects of spontaneous and hypercapnic
hyperventilation on inspiratory effort sensation in normal subjects. Am J Respir Crit Care Med. 1998;158:107-10. https://doi.org/10.1164/ajrccm.158.1.9709098
25. Evans KC, Banzett RB, Adams L, McKay L, Frackowiak
RS, Corfield DR. BOLD fMRI identifies limbic, paralimÂbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;883:1500-11. https://doi.org/10.1152/jn.2002.88.3.1500
26. Mahler DA.
Understanding mechanisms and documentÂing plausibility of palliative
interventions for dyspnea. Curr Opin Support Palliat
Care. 2011;5:71-6.
http://dx.doi.org/10.1097/spc.0b013e328345bc84
27. Parshall MB, Schwartzstein RM,
Adams L, et al. An Official American Thoracic Society Statement: Update on the
Mechanisms, Assessment, and Management of Dyspnea. Am Jf
Resp Crit Care Med. 2012;185:435-52. https://doi.org/10.1164/rccm.201111-2042ST
28. Carrieri-Kohlman V, Donesky-Cuenco
D, Park SK, Mackin L, Nguyen HQ, Paul SM. Additional
evidence for the affective dimension of dyspnea in patients with COPD. Res Nurs Health. 2010;33:4-19. https://doi.org/10.1002/nur.20359
29. Banzett RB, Pedersen SH, Schwartzstein
RM, Lansing RW. The affective
dimension of laboratory dyspnea: air hunger is more unpleasant than
work/effort. Am J Respir Crit
Care Med. 2008;177:1384-90.
https://doi.org/10.1164/rccm.200711-1675OC
30. Scano G, Gigliotti F, Stendardi L, Gagliardi E. DysÂpnea
and emotional states in health and disease. Respir
Med. 2013;107:649-55.
https://doi.org/10.1016/j.rmed.2012.12.018
31. Davenport PW, Reep RL, Thompson FJ. Phrenic nerve
afferent activation of neurons in the cat SI cerebral corÂtex. J
Physiol. 2010;588t:873-86. https://doi.org/10.1113/jphysiol.2009.181735
32. Casey KL.
Forebrain mechanisms of nociception and pain: Analysis through imaging]. Proceedings of the NaÂtional Academy of Sciences. 1999;96:7668-74. http://dx.doi.org/10.1073/pnas.96.14.7668
33. De Peuter S, Van Diest I, Lemaigre V, Verleden G, Demedts M, Van den Bergh O. Dyspnea: the role of
psychological processes. Clin Psychol
Rev. 2004;24:557-81.
https://doi.org/10.1016/j.cpr.2004.05.001
34. Santiago TV,
Edelman NH. Opioids and breathing. J Appl Physiol. 1985;59:1675-85.
https://doi.org/10.1152/jappl.1985.59.6.1675
35. OâDonnell DE, Ora J, Webb KA, Laveneziana P,
Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol. 2009;167:116-32.
https://doi.org/10.1016/j.resp.2009.01.010
36. Marciniuk DD, Goodridge D,
Hernandez P, et al. Managing dyspnea in patients with advanced chronic
obstructive pulmonary disease: a Canadian Thoracic Society clinical practice
guideline. Can Respir J. 2011;18:69-78.
https://doi.org/10.1155/2011/745047
37. Comroe JH, Foster RE, Dubois AB, Briscoe WA, Carlsen E. El pulmÃģn. FisiologÃa clÃnica y pruebas
funcionales pulmonares. Editorial Universitaria; 1964.
38. Comroe JH. Dyspnea.
Mod Concepts Cardiovasc Dis. 1956;25:347-9.