Autor : Baloco, Oscar1, SÃvori, Martin2, Jajati, MÃģnica1, Serrano, Mariela1, GonzÃĄlez, Claudio1, Rey, DarÃo3
1Pulmonology University Center Dr. J. M. Ramos MejÃa, Pulmonology and Tisiology Unit, Hospital Dr. J. M. Ramos MejÃa, Faculty of Medicine, University of Buenos Aires (UBA), Autonomous City of Buenos Aires. 2Pulmonology University Center Dr. J. M. Ramos MejÃa, Faculty of Medicine, University of Buenos Aires, director of the Pulmonology Specialization Career, Faculty of Medicine, UBA. 3Pulmonology University Center Dr. J. M. Ramos MejÃa, Faculty of Medicine, University of Buenos Aires, Director of the Pulmonology Specialization Career, Hospital General de Agudos Dr. Enrique TornÚ.
https://doi.org/10.56538/ramr.EZRJ2537
Correspondencia : Oscar Baloco. Urquiza 609, CABA (1405). E-mail: oscarbalocoe@hotmail.com
Received: 09/02/2023
Accepted: 11/02/2023
The endothoracic
fascia and the extrapleural space are sites of
pathological processes closely linked to the pathology of the lung parenchyma
and pleura, including tuberculosis (TB).1-2
Understanding the anatomical structure will prevent the confusion
that normally occurs with pleural localization, since the pathogenesis and some
diagnostic methods differ from pleuropulmonary forms
of tuberculosis, as well as the duration of treatment.1-2
The first refÂerence is from Boyer in 1846, but Wunderlich in 1861 reported the initial description as âperipleuritis,â hence it has been known as the âWunderlichâs disease.â1
In 1867 Billroth and Verneuil emphasized the importance of the lymph nodes in the
pathogenesis of this presentation.1 Kauffmann
supported the lymphatic pathogenesis of cold abscesses of the thoracic wall.1 In 1939, Skarby provided a brilliant radiological work that was
fundamental for unÂderstanding this condition.3
In our country, the first description of 30 cases of tuberculous peripleuritis was in
1945, by Prof. O. Vaccarezza.4 So peripleuritis was defined as âthe inflammation (of
different degrees) of the tissues located between the parietal pleura and the
thoracic wall.â4 It is also
known by other synonyms such as cellulitis or endothoracic
fasciitis, epipleuritis, peripleural
abscess, or cold abscess in the Anglo-Saxon literature.1-2
The main cause of peripleuritis
is infectious, with Mycobacterium tuberculosis being the most common
bacterium. However, other etiologies have been confirmed, such as fungal
infections by Paracoccidioides brasiliensis and Actinomyces
israelii, as well as other non-infectious causes
like lymphomas, myelomas, benign or malignant tumors, and trauma.1-2
The objective of this
communication was to understand the incidence and demographic characteristics
of tuberculous peripleuritis
(TBPP), its associaÂtion with parenchymal lesions, its coinfection
with HIV, and the compliance and treatment withdrawal rates recorded in a
public hospital during the period 1983-2021.
MATERIALS AND METHODS
A retrospective analysis was
conducted on cases of TBPP that were confirmed through biopsy or imaging and
were reported through the respective program form and docuÂmented in the
medical records archive and the computer system of the Ministry of Health of
the Government of the Autonomous City of Buenos Aires (SIGEHOS).
The following data were
considered: demographic backÂground, BCG (Bacille Calmette-GuÃĐrin) vaccination history, coinfection with HIV, presence or absence of
concurrent lung involvement, treatment adherence, toxicity, pharmaÂcological
resistance, and mortality.
RESULTS
4,076 cases of TB were reported,
11 of which were TBPP (0.27 %, or an incidence of 269.8 cases of TBPP per
100,000 cases of TB).
The median age was 42 years (IQR,
interquartile range of 23-96); and males accounted for 72.7 % (n = 8) of the
sample. 36.3 % (n = 4) had been BCG-vaccinated, and 18 % (n = 2) had coinfection with HIV (from 1989 to 2021).
With regard to pulmonary
presentation, 54.5 % (n = 6) of the patients showed associated paÂrenchymal
lesions: 50 % (n = 3) had solitary non-cavitary
lesions, and the rest included: 1 with solitary cavitary
lesions, 1 with bilateral non-cavitary lesions, and 1
with bilateral cavitary lesions (Figure 1).
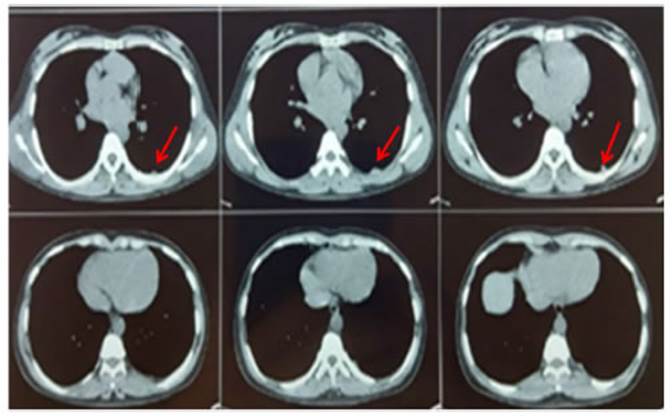
The diagnosis was confirmed by
biopsy in 72.7 % (n = 8) of the cases and was clinically and radiologically determined in the remaining cases. The
anatomical areas involved were the internal mammary lymph node chain in 63 % (n
= 7), the paravertebral in 27 % (n = 3), and intercostal in 9 % (n = 1) (Figure
2).
Seven patients (63.6 %) completed
treatment, three patients (27 %) discontinued treatment, and one patient (9 %)
passed away during the treatment. In terms of follow-up, one patient showed
rifampicin resistance (patient with conÂcurrent lung involvement), and another
patient (9 %) experienced reversible hepatotoxicity due to pyrazinamide.
DISCUSSION
A case series of TBPP has been
described in a multispecialty public hospital in the city of BueÂnos Aires. Its
incidence was extremely low. It predominated in middle-aged men without BCG
vaccination and with non-cavitary unilateral lung
involvement. Coinfection with HIV was considerÂable,
which could be attributed to the profile of our institution. In almost three
out of four patients, a diagnosis was made through biopsy, predominantly
involving the internal mammary chain. Treatment withdrawal was reported in at
least one every four patients, which was associated with polysubstance
consumption and homelessness.
Although TBPP was first described
in the mid-19th century, in our country, it was Prof. O. Vaccarezza
who first described his series of 30 cases.1,4 Defined as
âthe inflammation (of different degrees) of the tissues located between the
parietal pleura and the thoracic wall,â it is often an underdiagnosed form of
presentation of TB.1-2 Some years
later, Professors Juan Carlos Rey and Pedro Rubenstein described another case
series.5-6
To understand its clinical
presentation, it is necessary to recognize the normality of the periÂpleural anatomical space.1,2 The endothoracic fascia is an anatomical structure consisting
of the apposition of numerous fibrils oriented in different directions and in a
heterogeneous way. This explains how within the endothoracic
fascia, purulent collections can adopt different arrangeÂments and sizes. The
lymph nodes of the thoracic wall are divided into four groups: 1) Posterior
parietal nodes located in the costovertebral angle
within the thickening of the endothoracic fascia; 2)
Anterior or internal mammary nodes, which are also situated in the
corresponding thickening of the fascia; 3) Intercostal nodes located between
the intercostal muscles and the lateral wall of the thorax, directly receiving lymphatics from the parietal pleura; 4) Diaphragmatic
nodes.1,2 Given
the heterogeneous arrangement of the fibrils, weak points are generated in the
thoracic wall, allowing the opening of peripleuritic
abscesses from the inside to the outside. They herniate and lead to the
formation of cold abscesses. These can be intermuscular,
emerging over the anterolatÂeral areas of the chest, or intramuscular, followÂing
the paths of the perforating nerves. Others may become externalized as caseous-purulent forms.1-2,7-8 Tuberculous peripleuritis can
originate in the pulmonary parenchyma, the pleura, or the thoracic wall. The
process of primoinfection that develops in the lung,
from the bacillus nesting in the alveoli to the primary infiltration, allows
the observation of the contamination of the pleural serosa.1-2,7-8 In the
secondary period of Ranke, the hematogenous route and
contiguous spread through traumatic or iatrogenic means would be the mechanism
of pleural infection, the starting point of peripleuritis
when the lymph node in the extrapleural zone becomes
infected, resulting in tuberculous adenitis. In the
early stages of Ranke, peripleuritis manifests in its
caseous-purulent form, while in the tertiary period,
the fibrosclerotic form predominates.1-2,7-8 In chest
images, it can apÂpear as juxtacostal radiopacities or areas of higher attenuation in tomographies, with the free edge directed towards the lung,
convex in its central portion, and concave at both ends (Skarby
Sign).3
Despite the low incidence of
presentation comÂpared to more traditional forms of TB, our series of TBPP has
a significant number of cases in modern times if compared with the number
reported on a national level more than 50 years ago, especially considering
that most of those cases were diagÂnosed in the pre-antibiotic era of TB
treatment.4-6
In conclusion, in this report,
the incidence of TBPP was extremely low among patients diagÂnosed with TB,
predominantly in middle-aged men without BCG vaccination and with non-cavitary unilateral lung involvement. Coinfection
with HIV was considerable (18 %), and this could be attributed to the profile
of our institution. A case of TBPP had already been reported in a patient with
HIV.9 In almost three out of four patients, a diagnosis was made
through biopsy, the preferred procedure in these cases, mostly involving the
internal mammary chain. Treatment withdrawal was reported in at least one every
four patients, which was associated with polysubstance
consumpÂtion and homelessness. Due to the significant incidence of TB in our
country and the low clinical-radiological suspicion of peripleuritis,
this form of clinical presentation should be considered for early diagnosis and
treatment.
REFERENCES
1.
Corti M, VillafaÃąe MF, Palmieri O, Negroni R, Millet G. Algunas consideraciones acerca de las afecciones
del espacio peripleural. Rev
Argent Radiol 2005;69:153-6.
2.
Rey D. Consideraciones sobre afecciones de la fascia endoÂtorÃĄcica
o peripleura. Rev Am Med Resp 2021;21:415-8.
3.
Skarby HG. Ãber die diagnostik extrapleuralÂer abszesse. Acta Radiol 1938;19:259-72. https://doi.org/10.3109/00016923809137763
4.
Vaccarezza OA. Consideraciones sobre la peripleuritis tuÂberculosa. Anales de PatologÃa y ClÃnica
de la Tuberculosis 1945;Tomo VII. N° 2:237.
5.
Rey JC, Rubinstein P. Consideraciones sobre un caso de peripleuritis
tuberculosa en el curso de una primo infecciÃģn. Rev Asoc Med Argent. 1947; 61: 619-20.
6. Rubinstein P, Rey DR. Peripleuritis (5 observaciones) Trib Med 1974, XIX: 368-79.
7. Myers J. The
natural history of tuberculosis in the human body. JAMA 1965;194:184-90. https://doi.org/10.1001/jama.1965.03090230054013
8.
Mutafian RV, Goldstein A, Rezzonico G, Monteleone P. Peripleuritis tuberculosa. Arch Arg Pediatr 1984;82:126-31.
9.
Esquivel P, Palmieri O, Corti M..TumoraciÃģn de la pared anterior del tÃģrax en un
paciente con sida. Enferm Infecc
Microbiol Clin 2002;20:223-4. https://doi.org/10.1016/S0213-005X(02)72795-X