Autor : Luna, Carlos M.1, Pulido, Laura2, Rizzo, Oscar3, Gauna, MarĂa Laura4, Chirino, Alejandro5, Videla, Alejandro J.6
1 Department of Medicine, Pulmonology Division, Hospital de ClĂnicas, Faculty of Medicine, University of Buenos Aires, Buenos Aires, Argentina 2 Hospital Italiano de Rosario, Sanatorio Americano. Rosario, Santa Fe Argentina 3Hospital MarĂa Ferrer. University of Buenos Aires. Argentina 4Hospital Alberto Conacchiari Leandro N Alem, Buenos Aires. Sanatorio JunĂn, JunĂn, Buenos Aires 5Respira Salud ClĂnica Integral, Mendoza, Argentina 6Hospital Universitario Austral. Universidad Austral. Pilar, Argentina
https://doi.org/10.56538/ramr.BVIH9262
Correspondencia : Carlos M. Luna, address: Arenales 2557, 1st floor, Apartment A, zip code: 1425, Ciudad AutĂłnoma de Buenos Aires, Argentina, E-mail: dr.cm.luna@gmail.com
ABSTRACT
Adult smokers, subjects with
comorbidities, and the elderly are at higher risk of getting pulmonary
infections with worse outcomes. Community-acquired pneumonia caused by viruses,
pneumococcus, other bacteria, and “atypical” microorganisms affects both
healthy and sick adults. The influenza virus vaccine is designed the previous
summer and is oriented towards the strains that are expected for the following
season. Its efÂfectiveness depends fundamentally on the viral variant which is
ultimately responsible for the outbreak. The anti-pneumococcal polysaccharide
vaccine has been since 1983 and it is expected to be replaced by more effective
conjugate vaccines which prevent the infection due to the serotypes present in
the vaccine. Immunization against SARS-CoV-2 diminished contagion and severity
of COVID-19 remarkably. The acellular vaccine for Bordetella pertussis is not on the adult
schedule, despite the fact that vaccinating adults strengthens contagion
control in children. Double bacterial (diphtheria and tetanus), DTP (double +
pertussis), measles, varicella, rubella, HPV, Haemophilus
influenzae, meningococcal, herpes zoster,
Argentine hemorrhagic fever, and yellow fever vaccines are, but their use is
limited. New vaccines such as the one recently approved by the CDC (Centers for
Disease Control and Prevention) against respiratory syncytial virus will soon
be available.
Key words: Immunization, Influenza, Pneumococcus, Diphtheria, Tetanus, Herpes
zoster, COVID-19, Respiratory syncytial virus, Vaccines
RESUMEN
Los
adultos fumadores, con comorbilidades, y los ancianos tienen mayor riesgo de
contraer infecciones pulmonares y de tener peor evoluciĂłn. La neumonĂa
adquirida en la comunidad debida a virus, neumococo, además de otras bacterias
y microorÂganismos “atĂpicos” afecta tanto a adultos sanos como enfermos. La
vacuna antigriÂpal se diseña el verano anterior orientada
a las cepas esperadas para la temporada siguiente. Su eficacia depende
fundamentalmente de la variante viral que finalmente sea la responsable del brote.
La vacuna anti-neumocócica polisacárida
existe desde 1983 y será inexorablemente reemplazada por vacunas conjugadas de
mayor eficacia, que previenen la infecciĂłn por los serotipos presentes en la
vacuna. La inmunizaciĂłn contra SARS-CoV-2 acelerĂł la reducciĂłn del contagio y
la gravedad de COVID-19 notablemente. La vacuna acelular
para Bordetella pertussis
no está en el calendario de adultos, aun cuando vacunarlos fortalece el control
del contagio infantil. La vacunas doble bacteriana (difteria y tétanos), y
triple (doble + pertusis), y contra sarampiĂłn,
varicela, rubeola, HPV, Haemophylus influenzae, meningococo, herpes zóster, fiebre hemorrágica
argentina y fiebre amarilla están disponibles, pero son de uso limitado. Nuevas
vacunas, como la recientemente aprobada por los CDC contra el virus sincicial respiratorio, pronto estarán disponibles.
Palabras
clave: InmunizaciĂłn,
Influenza, Neumococo, Difteria, TĂ©tanos, Herpes zoster, COÂVID-19, Virus sincicial respiratorio, Vacunas
Received: 07/10/2022
Accepted: 08/25/2023
INTRODUCTION
The lung disease specialist
devotes part of his/her consultation time to review the vaccination history of
the patient. In 2015, the Argentine Association of Respiratory Medicine (AAMR) undertook
the inÂstitutional initiative to develop vaccination recomÂmendations for
adults with respiratory diseases.1 In recent decades, life expectancy and population age have
increased, leading to a higher percentÂage of patients with comorbidities and individuals
aged 65 and older (typical candidates for vaccinaÂtion). This group was between
10% and 19% of the population in 2015, and it is expected to be between 25% and
29% by 2050.2 In the eight
years since the publication of our recommendation, the COVID-19 pandemic
emerged, bringing significant advancements in vaccine development and notably
impacting upon the awareness of the importance of this preventive practice, its
perceived impact on disease prevention, and its morbidity and mortality
consequences.3
The emergence of SARS-CoV-2 as
the primary pathogen responsible for respiratory infections over the past three
years has shown the strengths and weaknesses of the vaccination programs. Along
with the approval of new vaccines worldÂwide in recent years, this compels us
to carefully reevaluate our perspective and update recommenÂdations on flu
vaccination (FV), anti-pneumococcal vaccination (APV), anti-COVID-19
vaccination (ACV), Bordetella pertussis vaccine
(BPV), herpes zoster vaccine (HZV), and respiratory syncytial virus (RSV)
vaccine.4
The Ministry of Health of the
Nation (MSAL) in Argentina, in a way similar to the practices of ofÂficial
public health agencies in other countries such as the Centers for Disease
Control and Prevention (CDC) in the United States or the European Center for
Disease Prevention and Control (ECDC) in Europe, provides vaccination
recommendations for people from birth and throughout their entire life.5
BACKGROUND
The MSAL has established a
national vaccination schedule comprising 17 vaccines recommended for children
starting from birth. This schedule includes explicit instructions regarding the
numÂber of doses, the time intervals between doses, booster shots, and
descriptions of some special vaccination schedules.5
It also specified differÂent vaccine
recommendations for certain at-risk population groups, including pregnant
women, postpartum women, and healthcare personnel. Interestingly, in the
schedule for individuals under 65 years without comorbidities, only the hepatitis
B and double bacterial vaccines are mentioned. For pregnant women, postpartum
women, and healthcare personnel, the same vaccines are recommended, adding
necessary doses of DTP and MMR vaccines. For individuals aged 65 or older, or
those under that age who have comorbidities, respiratory diseases, and smokers
at an increased risk of experiencing viral and bacterial pulmonary infections
and their consequences, the flu and anti-pneumococcal vaccines are included,
apart from those already mentioned.5 There are
SARS-CoV-2 vaccination schedules that are subject to changes depending on the
evolution of the pandemic, and these changes can be difficult to predict.
Community-acquired pneumonia
(CAP) occurs in more than 1% of individuals each year.6
PosÂsible pathogens of CAP include viruses, common bacteria,
intracellular or atypical bacteria, fungi, and protozoa.7
Infections caused by some of these pathogens can be prevented
through immunizaÂtion with vaccines. Therefore, the CDC in the United States recommend lifelong vaccination to provide immunity. However,
vaccination rates in adults worldwide are low.8
At the beginning of the 20th
century, the priÂmary cause of death worldwide was acute pulmoÂnary infection
(characterized as influenza and pneumonia). Advances in medicine significantly
improved life expectancy to around 80 years, and although pulmonary infections
were no longer a leading cause of death compared to cardiovascular diseases,
malignant tumors, unintentional injuÂries, and chronic respiratory diseases,
during 2020 and 2021, COVID-19 became the leading cause of mortality in many
countries, once again bringing infections to the forefront.9
VACCINATION AGAINST INFLUENZA
The Influenza (flu) is
characterized by annual epidemics that occur worldwide during the winter season
(between April and October in the southern hemisphere and between October and
April in the northern hemisphere). These epidemics can vary in severity. The
flu typically presents with an acute onset, with or without fever, and includes
general and respiratory symptoms that often improve within 7 to 10 days. In
some cases, especially in individuals older than 65 years and in adults and
children with chronic respiratory and cardiovasÂcular diseases, metabolic
diseases, renal failure, hemoglobinopathies, and
immunosuppression (including HIV+), medical attention or hospitalÂization could
be necessary, or even a fatal outcome could occur (10). Hence, the National
Vaccination Schedule of the Ministry of Health in Argentina recommends the influenza
vaccine starting from 6 months of age with no upper age limit for individuÂals
at a higher risk of complications from the flu. Vaccination is also recommended
for healthcare personnel, close contacts of immunosuppressed patients, and
individuals who work in close contact with live birds.10
The 2014 Vaccination Guideline of the MSAL already indicated
that: “Patients older than 65 years will not require medical prescripÂtion to receive
the flu vaccine”, in an attempt to reduce obstacles to achieving high
vaccination rates. We must remember the importance of anÂnual revaccination due
to the decline in antibody titers and the loss of vaccine efficacy as a result
of radical antigenic change in the hemagglutinin (H)
or neuraminidase (N) components (antigenic shift) and minor changes in the
structure of these proteins (antigenic drift) that the virus periodically
undergoes.11,
12
The CDC in the United States
simplified their influenza vaccination recommendation in 2010 (following the
H1N1 pandemic) to “every indiÂvidual of more than 6 months in the United States
should get a flu vaccine each season, with rare exceptions”13, clarifying that if vaccine supply is
limited, priority should be given to individuals older than 65 years,
individuals with comorbidiÂties, and contacts of people at increased risk. This
coverage limitation for cases of shortage is similar to the common
recommendation in Argentina.
Of the 4 recognized types of
influenza viruses, only types A and B currently cause epidemics in humans. Type
A is further divided into different subtypes based on its surface proteins H
and N. The two subtypes of Influenza A viruses circuÂlating at the time of
writing this document are A(H1N1) and A(H3N2).
Influenza B viruses are classified into two currently circulating lineages:
B/Victoria and B/Yamagata. The appearance of errors in the RNA-dependent
polymerase during coinfections between humans and
other species can modify the viruses, favoring the circulation of this variant
in a new host.14
Since the late 1970s, the
trivalent vaccine (containing two subtypes of influenza A
virus and one lineage of influenza B virus) has been. In Argentina, the
inactivated trivalent vaccine is and is directed towards the strain patterns
recomÂmended by the World Health Organization (WHO) for the relevant time
period (10). Furthermore, for the past few years, the quadrivalent
vaccine with two subtypes of A virus and with both
lineages of B virus has also been. There is also an inacÂtivated trivalent
vaccine with an adjuvant that enhances the immunization effect. An adjuvant is
an ingredient that helps create a stronger immune response, improving its
effectiveness, particularly in individuals aged 65 and older, individuals under
65 years with comorbidities and immunosupÂpressed subjects.15
Finally, in Argentina, we will also have the high-dose influenza
vaccine, recently approved by ANMAT (National Administration of Drugs, Food,
and Medical Technology). While most vaccines contain 15 μg of each H antigen, the high-dose vaccine contains 60 μg of each antigen.16 In August
2022, a feasibility study conducted in Denmark showed a 49% reduction in the
risk of death associated with the high-dose vaccine and also demonstrated a 64%
reduction in the incidence of hospitalization due to influenza or pneumonia
compared to standard-dose vaccination in older adults.17
Additionally, it’s important to note that most inactivated
influenza vaccines are manufacÂtured through the method of influenza virus culÂtivation
in embryonated eggs. However, there are also some
vaccines produced from cell lines, which offer significant advantages such as
the ability to produce larger quantities of vaccines more quickly. Cell-based
vaccines have the additional benefit of avoiding the possibility of mutations
that may arise during cultivation in embryonated eggs
and not requiring the use of egg proteins, which can be beneficial for certain
individuals with allergies or dietary restrictions.18
There are also live-attenÂuated (of intranasal application) and
recombinant vaccines, not currently in our country.10
In the year 2013, the first quadrivalent vaccine (two subtypes of A
virus and two lineages of B viÂrus) became12, with a good safety profile. Adding a
second B lineage to the influenza vaccine provides an increased immune response
to the additional subtype without reducing the immune response to the other
three subtypes or negatively affecting the safety and tolerance profile. By
offering broader protection against different lineages of influenza B virus
that co-circulate, the quadrivalent vaccine has the
potential to further reduce morbidity and mortality related to influenza beyond
what was achieved with trivalent vaccines.19, 20 Currently, in the United States and several
European countries, the quadrivalent vaccine has
replaced the trivalent vaccine.13,
15
The group of patients seen by
pulmonologists is generally characterized by having a high risk of suffering
complications from severe influenza.21
In adults, the influenza vaccination is recomÂmended for
individuals aged 65 and older and for those under 65 with chronic pulmonary
disease or cardiovascular, renal, hepatic, or neurological disease, metabolic
disorders, including diabetes, hemoglobinopathies,
and immunosuppression (including HIV+). This includes a vast majority of the
patients treated by a pulmonologist.
Furthermore, the seasonality of
influenza poses a challenge to determine the optimal vaccination timing in
Latin America. While in temperate climates like South America, there are peaks
of activity during the winter months, in tropical and subtropical regions,
influenza occurs throughout the year, especially during the rainy season.22 Hive et al
conducted an analysis based on influenza seasonality studies carried out by the
CDC and the WHO, among others, collecting data from 138 countries located
either wholly or partially between the 38th parallel north and south. They
concluded that the main influenza season in most South American countries is
between April and June.23
The efficacy and side effects of
the influenÂza vaccine are measured like any other drug through randomized,
double-blind clinical trials. Real-world effectiveness of authorized vaccines
is assessed through effectiveness studies. VacÂcine effectiveness is related to
age, the presence of comorbidities, and real-world coverage of the circulating
virus strains.
The effectiveness of the
influenza vaccine is calculated every year by the CDC.24
Figure 1 shows the effectiveness of the influenza vaccine
measured during 16 of the last 17 influenza seasons in the northern hemisphere
(it was not measured in the 2020-2021 season due to the low viral circulation
caused by the strict isolation measures of the pandemic).
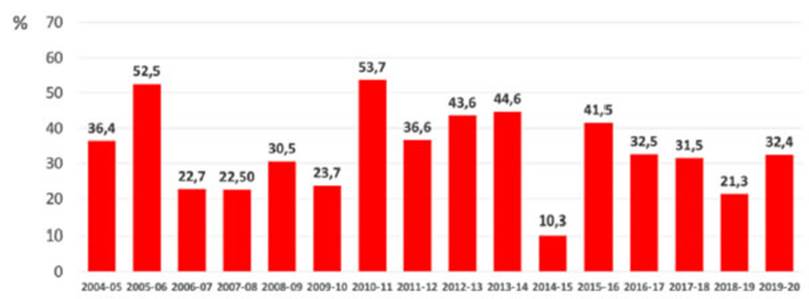
While the modest effectiveness of
currently inÂfluenza vaccines may be surprising at first glance, as seen
recently with the SARS-CoV-2 pandemic, the vaccine can prevent severe
complications of the influenza. This includes a 26% reduction in ICU admissions
and a 31% reduction in mortalÂity among adults with influenza associated with
hospitalization admission.25,
26
ANTI-PNEUMOCOCCAL VACCINATION
Before 1945, pneumonia in adults
was caused in over 90% of cases by Streptococcus pneumoniae.
However, starting from 1950, the proportion of pneumonia cases caused by this
organism began to decrease. Currently, the pneumococci are presÂent in less
than 10%-15% of the cases, with this proportion being higher in Europe, likely
due to differences in vaccination practices and smoking habits. Other pathogens
such as gram-negative bacilli Staphylococcus aureus,
Chlamydia, MycoÂplasma, and Legionella are identified in a range
of 2% to 5% of patients hospitalized for pneumoÂnia. Viruses are found in 25%
of patients, and approximately one-third of them have bacterial coinfections. It is important to note that in more than 50%
of the cases, recent studies have failed to identify the causative organism,
which remains the primary challenge in understanding lower respiratory infections.27
The 2023 Vaccination Schedule in
Argentina does not specify indications for the anti-pneuÂmococcal vaccine
(APV); it only recommends a sequential schedule for adults. In the document
“Technical Guidelines and Vaccinator Manual for Pneumococcal Vaccination,
2017-1028 TechÂnical Strategy”, the MSAL indicates that the APV should be
administered to adults who are at high risk of invasive disease, including
sickle cell anemia, congenital heart disease, chronic lung diseases, diabetes
mellitus, chronic liver disease, cerebrospinal fluid fistula, functional or
anatomiÂcal asplenia, cochlear implant, HIV
infection, leuÂkemia, Hodgkin’s and non-Hodgkin’s lymphomas, multiple myeloma,
other neoplasms, chronic renal failure, nephrotic
syndrome, chemotherapy or corticosteroid treatments, and organ transplants.
Regarding revaccination, it states that individuals at high risk of invasive
disease, such as functional or anatomical asplenia,
chronic renal failure, neÂphrotic syndrome, HIV
infection, transplantation, leukemia, lymphoma, multiple myeloma, other
neoplasms, and immunosuppressive treatment, can receive one revaccination with
the 23-valent pneumococcal polysaccharide vaccine (PPSV23). It also suggests
that high-risk pregnant women who have not previously received the APV can
receive it starting from the 16th week of gestation. It is advisable to take
the APV, if applicable, when receiving the annual FV. Individuals aged 65 and
older do not require a doctor’s prescription to be vaccinated.28
Expert Consensus Document of the
Latin American Thoracic Association (ALAT) and the Spanish Society of
Pulmonology and Thoracic Surgery (SEPAR), concluded that tobacco consumption is
a highly significant risk factor for the development of pneumococcal disease in
its clinical forms of community-acquired pneumonia (CAP) and invasive
pneumococcal disease (IPD).29
In our region, we have had the
PPSV23 since 1983 (covering serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A,
11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) and the 13-valent
pneumococcal conjugate vaccine (PCV13) (covering serotypes 1, 3, 4, 5, 6A, 6B,
7F, 9V, 14, 18C, 19A, 19F, and 23F), conjugated with the CRM 197 carrier
protein, since 2013. PCV13 was approved by ANMAT for individuals over 50 years
of age in 2012 based on its higher immunogenicity for 10 of the 12 shared
vaccine serotypes.30 Pneumococcal
vaccines are administered sequentially (PCV13 followed by PPSV23) as
recommended by the Advisory ComÂmittee on Immunization Practices (ACIP) in the
United States for individuals older than 18 years with risk factors, including
candidates and reÂcipients of hematopoietic cell transplantation. In 2014, the
ACIP started to recommend the PCV13 for adults aged ≥ 65
years.31
In 2015, Bonten
et al published a double-blind, randomized, placebo-controlled parallel-group
study that strikingly demonstrated that in vacÂcinated individuals, a single
dose of the PCV13 vaccine results in a 45.6% decrease in the amount of first
episodes of CAP caused by any of the serotypes present in the vaccine (p <
0.001); a 45.0% decrease in the amount of episodes of non-bacteremic/non-invasive
CAP due to a serotype present in the vaccine (p = 0.007); and a 75.0% reduction
in the number of first episodes of IPD (p < 0.001).(p < 0.001).31,32 A narrative
report of Dunne et al compared the effectiveness of the PPSV23 and PCV13
vaccines in the same adult populations. They found that vaccine effectiveness
varies between 10 and 11% for PPSV23, between 40 and 79% for PCV13, and 39 to
83% for the PCV13/PPSV23 vaccines. Vaccine effectiveness against pneumonia (of
all causes) or lower respiraÂtory tract infection varies between 8 and 3% for
PPSV23 and between 9 and 12% for PCV13. These data confirm that the conjugate
vaccine has higher efficacy in preventing lung infections in adults.33 The latest
addition is the 20-serotype conjugate vaccine (PCV20), which is indicated for
active imÂmunization to prevent pneumonia and invasive diseases caused by
serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F,
22F, 23F, and 33F of Streptococcus pneumoniae in
adults older than 18. The PCV20 vaccine approved by the FDA (Food and Drug
Administration) in 2021 and by the EMEA (European Medicines Agency) in 2022, was approved by ANMAT in mid-June 2023. PCV20 adds 7
new serotypes to the 13-valent vacÂcine, which could simplify vaccination
schedules in the near future.34,35
The currently recommended
pneumococcal vacÂcination regimen for immunocompetent
adults in Argentina involves the sequential administration of the PCV13
vaccine, followed by the PPSV23 vaccine 12 months later. The PPSV23 requires a
second dose after the age of 65 (provided that at least 5 years have passed
since the first dose). In immunosuppressed patients, the sequential
administration of PCV13 should be followed by PPSV23 at least 8 weeks later.28 Under special
circumstances (such as individuals who have had a splenectomy,
those with sickle cell anemia, or those with cerebrospinal fluid fistula), it
is recommended to administer a second dose of PPSV23 5 years after the first
dose, and in these cases, eventually a third dose could be administered after
the age of 6536 (Figure
2). Vaccination using the 20-valent vaccine suggests a much simpler schedule,
as shown in Figure 3.
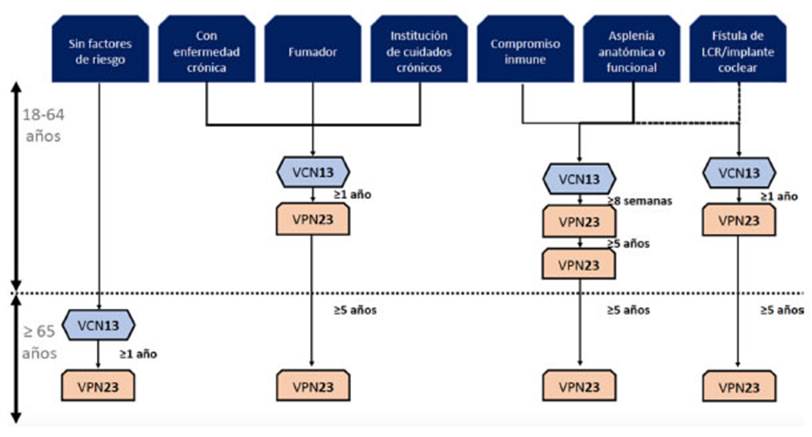
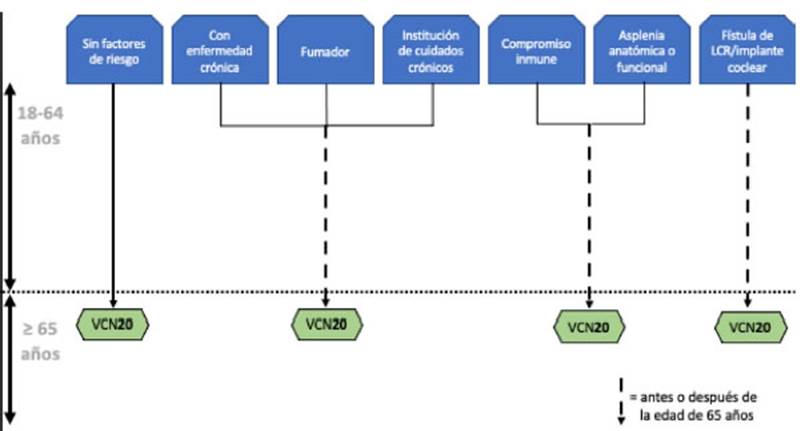
COVID-19 VACCINES
In November 2019, the appearance
of a new aniÂmal-origin coronavirus capable of causing severe respiratory
infections was reported in China, and the WHO declared it a pandemic in March
2020. Early that year, the virus was sequenced, enabling the rapid development
of vaccines based on viral vectors and messenger RNA platforms. These vacÂcines
have proven to be highly effective against severe forms of the disease in
clinical trials and real-world settings, both in adults and in pregnant women,
children, and adolescents.3,37 It is estimated
that the vaccines prevented the death of 19 million people in their first year
of use.38 Complete
vacciÂnation schedules provide protection ranging from 88% to 55% depending on
the variant of interest, with the highest effectiveness against the Alpha
variant and the lowest against Omicron.39
In our country, the vaccination campaign using initially vaccines
(Sputnik V, ChadOx1, and BBBIBP-CorV) was associated
with significant reductions in infecÂtion and mortality.40,41 When mRNA-based vaccines started to be used in
children and adolescents, a measurable benefit was also obtained.42 Evidence
gathered from systematic reviews suggests that mRNA platform-based vaccines are
associated with greater protection against the symptomatic disease.43 Observational
studies also show a deÂcrease in the risk of developing the symptoms of the
post-infection syndrome, also known as “long COVID” among vaccinated
individuals.44 It was also
shown that heterologous vaccine schedules provided protection, and this
strategy was used in some segments of the vaccination campaign.45
The ancestral virus was replaced
by new variÂants with higher contagion rates. In November 2021, the Omicron
variant was identified. Initially developed vaccines showed reduced
neutralizing capacity towards this new variant, and immunity declined more
rapidly.46 The
administration of booster doses showed the additional effectiveness of a third
dose compared to the primary series and of a fourth dose compared to the third,
mainly in people over the age of 60.47, 48 A 47% decrease in effectiveness against
infections was observed at 280 days, with 75% effectiveness at 112 days to
prevent hospitalization and death.49 According to
the Nominalized Federal Vaccination Registry, in Argentina, in May 2023, 9,314,083
people aged 50 or older had not yet received a booster dose in the last 6
months.50
In early 2022, bivalent vaccines
targeting both the ancestral variant and the new sub-variant were introduced
simultaneously.51,
52 These
vacÂcines have become the standard; however, eviÂdence shows that the
neutralizing antibody titers they induce are similar to those of monovalent
vaccines.53 Observational
studies have confirmed that administering bivalent boosters provided 50%
additional effectiveness to the doses of the primary series for individuals
aged 50 to 64 who had previously received two doses. In contrast, the
additional effectiveness was 22% for those over 65 who had received more than
two doses.54 The
explanation for this lower comparative efÂfectiveness of bivalent vaccines is
“imprinting” a phenomenon in which the immune response is primarily configured
against the antigens of the original variants presented by the first vaccines,
and an equally efficient response is not achieved when other antigens are
presented later. 55
Evidence shows that stronger
immunity is achieved when vaccine-induced immunity is combined with natural
immunity from infection, a situation that is very common considering the high
prevalence of infection in the communities.56
The recommendations of the MSAL that were in force at the time of
writing this document state that individuals of groups at high-risk of developÂing
severe forms of the disease (individuals aged 50 and older, immunosuppressed
individuals, and pregnant women) should receive a COVID-19 booster dose 6
months after their last dose, reÂgardless of the number of previous booster
doses received and with a minimum interval of at least 4 months since the last
dose. People under 50 years old with comorbidities (chronic
illnesses and obesity) and those at higher risk of exposure (healthcare
personnel) and with strategic roles fall into the group at “medium-risk” of
experiencing severe illness or death from COVID-19 infection. The
recommendation for this group is to receive a new booster dose 6 months after
the last dose received and, subsequently, they should receive a booster dose
annually.
Individuals considered to be at
low risk of sufferÂing complications (people under 50 years without comorbidities)
have access to COVID-19 booster vaccination, and it is recommended that they reÂceive
it annually.50
The most common adverse effects
of the vacÂcination are mild local reactions.57
The most concerning adverse effects
reported have been the occurrence of thrombosis (primarily associated with the
ChadOx1 vaccine) and myocarditis (linked to mRNA platform vaccines). While
modest increases in the risk of hematological and vascular events after
vaccination have been observed, this risk is much higher and prolonged for the
virus infection itself.58,
59 Myocarditis
is a very rare event, occurring in 1.08 cases per 100,000 vaccinated
individuals, and one every five events is severe.60
In this case as well, myocarditis from SARS-CoV-2 infection is
more common than myocarditis from vaccination, resulting in a risk-benefit
ratio highly favorable for vaccination.61
VACCINES AGAINST PERTUSSIS
Pertussis, also known as whooping
cough, is caused by the bacterium Bordetella
pertussis. The clasÂsic respiratory disease is characterized by three
stages: catarrhal, paroxysmal, and convalescent.62
During the catarrhal stage, infected
individuals experience rhinitis (inflammation of the nasal mucous membranes),
occasional mild cough, and low-grade fever. The paroxysmal stage is characterÂized
by spasmodic coughing, post-cough vomiting, and inspiratory wheezing. Symptoms
gradually improve during the convalescent stage, which typically lasts from 7
to 10 days but can extend for months. Factors influencing the clinical presentaÂtion
of whooping cough include age, immunity level, vaccination history, and the use
of antibiotics at the onset of the disease.63
The MSAL recommends combined
vaccination against pertussis, tetanus, diphtheria, HaemophiÂlus
influenzae type b, and Hepatitis B for infants
and young children, through a series of 4 doses of the 5-in-1 or pentavalent vaccine, including diphtheria and tetanus
toxoids and acellular perÂtussis (Argentina has the
cellular component ). This is followed by a dose of DTP (cellular triple
bacterial) which is administered at school admisÂsion at 5 years of age.5 Furthermore,
the ACIP recommends a booster dose at age 11 of the Tdap
vaccine (cellular triple bacterial vaccine with acelÂlular
pertussis, reduced diphtheria toxoid, and tetanus toxoid). Adults aged 19 to 64
who have never received the Tdap vaccine are also
advised to receive a booster dose. During pregnancy, it is recommended that
women receive a dose of Tdap after the 20th week,
preferably between weeks 27 and 36, regardless of whether they have previously
received this vaccine or not. After receiving the Tdap
vaccine, adolescents and adults are suggested to receive a booster dose of the
Td vaccine (tetanus and diphtheria toxoids) every 10 years to ensure ongoing
protection against tetanus and diphtheria and reduce the transmission of the
latter.5, 64
Recently, the GOLD (Global
Initiative for Chronic Obstructive Lung Disease) guidelines have recommended Tdap vaccination for patients with chronic obstructive
pulmonary disease who haven’t been vaccinated during adolescence.65
VACCINE AGAINST HERPES ZOSTER
Herpes Zoster (HZ) is a neurocutaneous disease produced by the reactivation of the
primary inÂfection of the varicella-zoster virus (VZV). This reactivation
results in chickenpox and the lifelong residence of the VZV genome in the
dorsal root or cranial nerve ganglia.66
The incidence and severÂity of HZ
increase with age. More than 90% of patients over 50 years old worldwide have
been infected with VZV and are therefore at risk of deÂveloping HZ, with an
incidence of approximately 2-4.6 people per 1,000 individuals per year. 67-70 The incidence
significantly increases in adults older than 80 years, reaching values between
10 and 12.8 cases per 1000 persons-year.71
This implies that one every three individuals between the ages of
50 and 90 will experience some episode of HZ (72). ImmuÂnity to HZ, initially
acquired innately (primoinfecÂtion) in children and
young adults when they get chickenpox can be maintained either intrinsically or
through extrinsic boosting. Intrinsic immunity is a subclinical response to
reactivation of VZV, while extrinsic boosting happens asymptomatically through
exposure to VZV in the community.72,79 The risk of HZ
increases in individuals aged 50 and older due to the effects of immunosenescence (cellular aging) or at any age due to
immunosupÂpression caused by various conditions such as HIV, diabetes mellitus,
COPD, chronic kidney disease, cardiovascular disease, among others, and/or by
immunosuppressive treatments like chemotherapy in the oncological population,
transplants, rheuÂmatologic diseases, or interstitial lung diseases (70). HZ
occurs when VZV-specific cell-mediated immunity fails to contain viral reactivation,
preÂsumably for being below a not-yet defined protecÂtive threshold, and the
reactivated VZV continues to spread.73, 74 HZ has multiple complications, with postherpetic neuralgia (10-15%) being the most common. It
is characterized by chronic radicular pain that can persist for more than 3
months after the vesicular eruption of HZ, and it can become disabling and very
difficult to treat. Chronic pain from postherpetic
neuralgia can affect between 5% and 30% of individuals who develop HZ, with a
higher frequency in older individuals, especially those over the age of 60.70, 75 If it affects
the eye reÂgion, especially after ophthalmic HZ, it can lead to complications
such as keratitis, conjunctivitis, and even glaucoma, which can scar and leave
severe lesions, including various degrees of blindness. In patients with severe
immunosuppression, there can be serious and highly fatal complications such as
disseminated HZ, myelitis, encephalitis, and/or cranial nerve paralysis.76 A previous version of the document recommended the live
attenuated vacÂcine against HZ containing the Oka VZV strain. This vaccine is
authorized for use in adults aged 50 and older. Medical literature has shown
that this vaccine has 51.3% effectiveness in preventing HZ and 66.5% effectiveness
in reducing postherpetic neuralgia in people aged 60
or older. However, its efficacy decreases with age, dropping from 69.8% in
adults aged 50-59 to 37.6% in those aged 70 or older. Furthermore, it is
contraindicated in individÂuals with immunosuppression, as live attenuated
vaccines could cause illnesses in this population.77
In 2015, a new recommendation was introduced, and in 2017, the
FDA voted unanimously on the recommendation of a new recombinant vaccine that
contains an antigen a glycoprotein called gE (a
fragment of VZV) along with an adjuvant called AS01B to boost the immune
response. Two doses separated by 2-6 months have an efficacy of 97.2% in
reducing the risk of HZ in adults aged 50 and older.75, 77 The duration of its efficacy has been studied
over the years, showing it maintained an efficacy of 87.9% for HZ prevention 4
years after having received two doses.71
Many individuals with asthma and almost all patients with COPD,
idÂiopathic pulmonary fibrosis, and other chronic lung conditions are older
than 50 years, the age at which this vaccine should begin to be used.1 Given the
fact that adults aren’t normally used to vaccine compliance monitoring,
pulmonoloÂgists can be considered one of the healthcare providers best suited
to recommend this vaccine as part of preventive healthcare measures for their
patients, positively influencing their atÂtitudes and beliefs.78
VACCINE AGAINST RESPIRATORY SYNCYTIAL VIRUS
The respiratory syncytial virus (RSV)
is a comÂmon cause of respiratory tract infections that can lead to severe
illness, often affecting infants and older adults. During the winter months, an
estimated 700,000 to two million cases of RSV are reported. In the United
States, RSV infections in older adults account for approximately 177,000
hospitalizations and 14,000 deaths annually.79
In Argentina, there are approximately 7,000,000 adults aged 65
and older; about 0.2% of them are expected to experience severe RSV infections,
which amounts to around 14,000 patients on averÂage.80
Pneumonia, COPD exacerbations or asthma require hospitalization
in patients who acquire severe RSV infection. 10% of these patients will die as
a consequence of the infection.81 In many cases, children are the source of infection for
older adults, while in other cases, the children can be infected by older
adults. Recently, the FDA has approved a vaccine against RSV for adults. The
effectiveness of this vaccine was 94.1% (95% confidence interval, 62.4 to 99.9)
against severe disease (pneumonia) and 71.7% (95% CI, 56.2 to 82.3) against RSV
causing exacerbation of asÂsociated respiratory disease.82 In Argentina, this vaccine has not yet been
submitted to ANMAT for approval; thus, it is not yet.
VACCINATION RECOMMENDATIONS FOR ADULTS FROM THE PULMONOLOGIST’S POINT OF
VIEW
Vaccines are important for
preventing diseases and disease complications throughout life. HowÂever,
vaccine coverage in adults is generally low and there is still a long way to go
before meeting established goals. Among the vaccines recomÂmended for adults,
the ones that stand out are those against influenza and pneumococcus, as they
aim to prevent respiratory complications. These vaccines are especially
important for paÂtients with chronic lung diseases such as COPD, asthma, and
chronic bronchitis. This committee proposes that, in addition to the specific
vaccinaÂtion schedule recommendations, healthcare proÂviders should follow the
guidelines of the Ministry of Health and other relevant organizations so as to
simplify adult vaccination recommendations according to different age groups,
always trying to complement such recommendations and not reduce them.
VACCINATION RECOMMENDATIONS FOR SPECIFIC DISEASES
Bronchial asthma
Asthma is a chronic respiratory
disease characterÂized by persistent inflammation where various types of cells
play a significant role. This inflammaÂtion causes symptoms in susceptible
individuals, associated with variable but widespread airflow obstruction, which
can be reversed spontaneÂously or with treatment. The inflammation also
increases bronchial hyperreactivity to various
stimuli.83 All asthmatic patients fall under the catÂegory of chronic
lung diseases, recognized among the annual FV recommendations and COVID-19
guidelines established by the MSAL and the 2022 Global Initiative for Asthma
(GINA) 9, 79. The CDC, through its 2030 immunization agenda (Healthy
People 2030), aims to reduce the morbidÂity and mortality of diseases that can be
prevented through vaccination and ensure access to new and existing vaccines
for the entire population. ImÂmunization is a global health success story.
Since 2010, more than 116 countries have introduced vaccines that they hadn’t
used previously, some of which are against deadly conditions like CAP 84.
A systematic review conducted by Boikos et al found a
positive association between asthma and IPD. This finding supports the idea of
considerÂing asthma a high-risk disease that requires the administration of
pneumococcal immunization. 85 Therefore,
basing on the cited information, expert consensus documents, the Spanish Asthma
ManÂagement Guidelines (GEMA 5.3), and the CDC, APV administration is
recommended for patients with asthma.86, 87
Chronic obstructive pulmonary disease
COPD is a heterogeneous disease
characterized by chronic respiratory symptoms (cough, dyspnea, sputum
production, and/or exacerbations) due to abnormalities in the airways and/or
alveoli that reÂsult in airway obstruction 65. Exacerbations are
primarily caused by viral infections that can sufÂfer bacterial superinfection, leading to increased inflammation and
originating symptoms and an impact on quality of life and survival. People with
COPD suffer from CAP more frequently, and COPD is associated with reduced
survival followÂing a CAP episode 88. COPD is a well-recognized
chronic lung disease listed among the annual FV and APV recommendations of the
National Guidelines for the Control of Vaccine-Preventable Diseases and the
Ministry of Health and Gender of the Autonomous City of Buenos Aires 89.
The GOLD guidelines, the Spanish COPD guideÂlines (GesEpoc),
and the national guidelines for COPD diagnosis and treatment from the MSAL
recommend the FV and APV with varying levels of evidence90, 91 The FV with inactivated viruses appears to
reduce the frequency of exacerbations and severe illness requiring
hospitalization.92,93 It is also effective in reducing the number of
inÂfluenza infections by 40% and could be associated with a lower risk of
ischemic cardiac events. 94,95 In terms of efficacy, evidence suggests that
the likelihood of not acquiring acute respiratory infecÂtions related to
influenza in individuals vaccinated with FV (trivalent, fragmented, and
inactivated virus) is 76%. In patients with mild, moderate, or severe COPD,
vaccine effectiveness was 84%, 45%, and 85%, respectively.96
Systematic reviews haven’t shown an effect of the FV on
mortality, the number of hospitalizations for influenza-like illness, or the
need for mechanical ventilation.92,
96 The
aforementioned guidelines agree on the APV recommendation for being one of the
comorbidiÂties included in the CDC’s recommendations. A systematic review by
the Cochrane CollaboraÂtion found that the APV reduces the incidence of CAP and
exacerbations in COPD patients.97 They also found that vaccination reduces the risk of CAP and
COPD exacerbations without an impact on the risk of confirmed pneumococcal
pneumonia.97,
98 Regarding
safety, reported local and systemic adverse effects are mild.92, 97, 98
Smoking
Evidence from population-based
studies indicates a higher risk of influenza-like illness (ILI) among smokers.
A survey conducted in 5943 individuÂals in Great Britain found that tobacco use
was one of the six factors associated with ILI in the multivariable analysis.99 FV and APV
are formally recommended for smokers, regardless of age, the presence of
comorbidities, or immune status, even in the absence of other risk factors.29, 100, 101
Other chronic lung diseases
Acute exacerbations of chronic
lung diseases are often associated with viral and bacterial pathogens. These
exacerbations contribute to lung function deterioration and poor quality of
life, and place an additional burden on individuÂals, families, communities,
and the healthcare sector. Therefore, preventing exacerbations is crucial in
clinical management. Several vaccines offer protection against respiratory
pathogens (Streptococcus pneumoniae, Bordetella pertussis, influenza, RSV, and SARS-CoV-2)
that can trigger exacerbations, but the evidence supporting their effectiveness
in the prevention of exacerbations of chronic lung diseases is limited.102 Much research focuses on other chronic lung diseases like
COPD, asthma, and cystic fibrosis. Bronchiectases reÂceive
less attention than other chronic respiraÂtory diseases in children and adults.
The role of existing vaccines against pathogens associated with the disease has
not been sufficiently studied, and the evidence of benefit is limited.103 NeverÂtheless,
due to the risk of complications such as severe pneumonia, IPD, and
hospitalization as a consequence of influenza, it is recommended that children
and adults with bronchiectasis (BQT) receive vaccines in accordance with the
national immunization program for high-risk groups.5
Additionally, it’s important to consider the role of maternal
immunization during pregnancy, as severe respiratory infections in early
childhood are associated with the development of bronchiÂectasis.
VACCINATION RECOMMENDATIONS FROM THE PULMONOLOGIST’S POINT OF VIEW
Taking into consideration the
patient’s history and age, supported by the availability of different vaccines,
the recommendations of the MSAL and recent publications, the Lung Infection
DepartÂment of the Argentine Association of Respiratory Medicine formulates the
following recommenÂdations for pulmonologists with regard to the vaccination of
adults with lung diseases.
Adults aged 18 to 65 years,
without a history of lung disease and without close contact with individuals at
high risk of comÂplications if they contract influenza:
They can receive an annual FV,
preferably the quadrivalent vaccine (containing two
strains of influenza A and two strains of influenza B), even if they do not
have a specific indication. They should be inquired about the last dose they
received of the Td vaccine, which should be repeated every 10 years. It is
recommended that one of the doses of the Td vaccine that are going to be
administered over their lifetime be given with the Tdap
vaccine if the patient has never been vaccinated with an acellular
pertussis vaccine. They can receive a primary series of the ACV and then an
annual booster dose.
Pregnant women
The FV is recommended during any
trimester of the pregnancy; and the Tdap vaccine is
recomÂmended after the 20th week of gestation in each pregnancy.
Adults aged 18 to 65 years, with
a history of lung disease, smoking, other comorbidiÂties, or contact with
high-risk patients who may experience complications if they conÂtract
influenza:
Annual FV, preferably the quadrivalent vaccine, and the APV according to the schedule
outlined in Figure 2. They should be inquired about the last dose they
received of the Td vaccine, which should be repeated every 10 years. It is
recommended that one of the doses of the Td vaccine that are to be administered
over their lifetime be given with the Tdap vaccine if
the patient has never been vacciÂnated with an acellular
vaccine against pertussis. They can receive the primary series of the ACV and
booster doses every 6 months.
Individuals older than 18 years
and youngÂer than 65 who have undergone a splenecÂtomy,
or those with sickle cell anemia, or cerebrospinal fluid fistula:
Annual FV, preferably the quadrivalent vaccine, and the APV according to the schedule
outlined in Figure 2. Td vaccine every 10 years. It is
recomÂmended that one of the doses of the Td vaccine that should be given over
their lifetime be administered with the Tdap vaccine
if the patient has never been vaccinated with an acellular
pertussis vaccine (Figure 1). They can receive the primary series of the ACV
and booster doses every 6 months.
Adults between 50 and 65 years
old, in good health:
Their schedule only recommends
the adminÂistration of the 2-in-1 Td vaccine every 10 years throughout their
life. It is recommended that one of the doses of this vaccine be administered
with the Tdap vaccine. Healthy individuals older than
50 years should be vaccinated against HZ. The recombinant vaccine is
administered in 2 doses separated by 2 to 6 months.
Individuals older than 65 years,
with or without comorbidities
The annual FV,
preferably the quadrivalent type, or the trivalent
type with adjuvant, and the APV according to the schedule outlined in Figure 2. Td vaccine every 10 years. It is recommended
that one of the doses of the Td vaccine that should be given over their
lifetime be administered with the Tdap vaccine if the
patient has never been vaccinated with an acellular
pertussis vaccine. If they haven’t been vaccinated against HZ yet, it is
recommended that they do so. They can receive the primary series of the ACV and
booster doses every 6 months.
Patients of any age admitted to
the intensive care unit with respiratory failure or heart failure
Vaccine against
herpes zoster. Annual FV, preferÂably
the quadrivalent type, and the APV according to the
schedule outlined in Figure 2. Td vaccine every 10 years.
It is recommended that one of the doses of the Td vaccine that should be given
over their lifetime be administered with the Tdap vacÂcine
if the patient has never been vaccinated with an acellular
pertussis vaccine. If they haven’t been vaccinated against HZ yet, it is
recommended that they do so. They can receive the primary series of the ACV and
then an annual booster dose.
Summary of recommendations in
Table 1
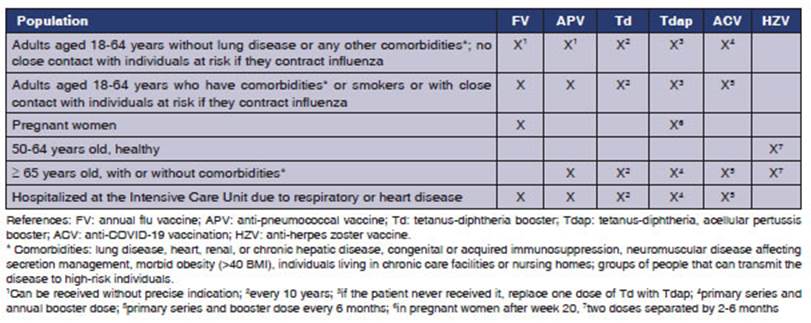
CONCLUSIONS
Chronic respiratory diseases are
a growing health problem, especially regarding tobacco consumption and the
aging of the population associated with the constant updates in immunization
and prophylaxis in all individuals with and without risk factors. This becomes
a paradox arising from advances in medical care over the last decades.
Under this scenario, the
pulmonologist plays a fundamental role as a guide, especially after the
emergence of COVID-19, where many specialties referred patients for
consultation on new vaccines, co-administration with FV and APV and other vaccines,
and time interval between doses, among other things.
Additionally, the pulmonologist
also faces the consequences of the lack of prevention of respiraÂtory
infections, such as exacerbations of COPD, of asthma, CAP, and other infectious
disorders. As a result, the pulmonologist is the specialist who prescribes and
informs the most about vaccines as the best method for the prevention of
infectious conditions in chronic diseases.
The objective of this writing
committee is to promote the constant updating of adult pulmonÂologists so that
they are prepared to play a direct role in the recommendation of vaccines to
their patients.
Conflict of interest
CML is a medical consultant for
Pfizer since 2012; he has participated in clinical trial protocols as an
investigator, adjudicator, or member of the Safety Monitoring Board for
Novartis, Boehringer, Bayer, and Pfizer in the past;
he has been a speaker for Pfizer since 2010. LP has participated as a speaker
for Glaxo on obstructive diseases in adults and as a
consultant for Merck Sharp & Dohme. AJV has
served as a consultant for Sanofi, GSK, Novartis, and
Pfizer. The rest of the authors have no conflicts of interest related to the
topic of this publication.
Document funding
This document is sponsored by SANOFI,
Pasteur, and SEQUIRUS laboratories.
REFERENCES
1.
Luna CM, Rizzo O, Monteverde A, et al. Recomendaciones de vacunaciĂłn en adultos
con enfermedades respiratoÂrias. Documento de la AsociaciĂłn Argentina de
Medicina Respiratoria para los neumonĂłlogos. Rev Am Med Resp. 2015;4:314-24.
2. Roser
M, Ortiz-Ospina E, Ritchie H. Life Expectancy. Our World in Data [Internet]. 2013 May 23 [cited 2023 Jun
5]; https://ourworldindata.org/life-expectancy
3. Shi T, Robertson C, Sheikh A. Effectiveness
and safety of coronavirus disease 2019 vaccines. Curr Opin
Pulm Med [Internet]. 2023;29(3):138-42.
http://dx.doi.org/10.1097/MCP.0000000000000948
4. Luna CM. Impact of vaccination
on the epidemiology and prognosis of pneumonia. Rev Esp Quimioter [Internet].
2022;35(Suppl 1):104-10.
http://dx.doi.org/10.37201/req/s01.22.2022
5.
Ministerio de Salud de la NaciĂłn, Argentina. Calendario Nacional de VacunaciĂłn
[Internet]. [cited 11 de
junio de 2023]. https://bancos.salud.gob.ar/recurso/calendario-nacional-de-vacunacion-2022.
6. Theilacker
C, Sprenger R, Leverkus F,
Walker J, Häckl D, von Eiff
C, et al. Population-based incidence and mortality of community-acquired
pneumonia in Germany. PLoS One [Internet]. 2021;16:e0253118.
http://dx.doi.org/10.1371/journal.pone.0253118
7. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-Acquired Pneumonia Requiring
Hospitalization among U.S. Adults. N Engl
J Med [Internet]. 2015;373:415-27.
http://dx.doi.org/10.1056/NEJMoa1500245
8. Vaccination coverage among
adults in the United States, national health interview survey, 2019-2020.
Centers for Disease Control and Prevention
9. COVID-19 Excess Mortality
Collaborators. Estimating excess mortality due to the
COVID-19 pandemic: a systematic analysis of COVID-19-related mortality,
2020-21. Lancet[Internet]. 2022;399:1513-36.
http://dx.doi.org/10.1016/S0140-6736(21)02796-3
10.
DirecciĂłn de Control de Enfermedades Inmunoprevenibles.
GuĂa Rápida VacunaciĂłn Antigripal. Ministerio de Salud de Argentina; 2023.
11.
Talledo M, Zumaeta K. Los virus Influenza y la nueva
pandemia A/H1N1. Rev Peru Biol [Internet]. 2009;16:227- 38.
http://www.scielo.org.pe/scielo.php?pid=S1727-99332009000200018&script=sci_arttext
12.
Carascal MB, Pavon RDN,
Rivera WL. Recent Progress in Recombinant Influenza Vaccine
Development Toward Heterosubtypic
Immune Response. Front Immunol [Internet]. 20229;13:878943. http://dx.doi.org/10.3389/fimmu.2022.878943
13. Centers for Disease Control
and Prevention. Recommended Adult Immunization Schedule for
ages 19 years or older [Internet]. U. S. Department of Health and Human
Services; 2023. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
14. A revision of the system of
nomenclature for influenza viruses: a WHO memorandum. Bull World Health Organ
[Internet]. 1980;58:585-91.
https://www.ncbi.nlm.nih.gov/pubmed/6969132
15. Scheduler EV. Vaccine
schedules in all countries in the European Union. May 27]
https://vaccine-schedule ecdc europa
eu. 2020;
16.
Robertson CA, DĂaz Granados CA, Decker MD, Chit A, Mercer M, Greenberg DP. Fluzone® High-Dose Influenza
Vaccine. Expert Rev Vaccines [Internet]. 2016;15):1495- 505.
http://dx.doi.org/10.1080/14760584.2016.1254044
17. Johansen ND, Modin D, Nealon J, Samson S, Salamand C, Larsen CS, et al. Feasibility of randomizing
Danish citizens aged 65-79 years to high-dose quadrivalent
influenza vaccine vs. standard-dose quadrivalent
influenza vaccine in a pragmatic registry-based setting: rationale and design
of the DANFLU-1 Trial. Pilot Feasibility Stud [Internet]. 2022;8:87. http://dx.doi.org/10.1186/s40814-022-01044-w
18. Kim EH, Kwon HI, Park SJ, Kim
YI, Si YJ, Lee IW, et al. Generation of a High-Growth Influenza Vaccine Strain
in MDCK Cells for Vaccine Preparedness. J Microbiol Biotechnol [Internet]. 2018;28:997-1006.
http://dx.doi.org/10.4014/jmb.1712.12007
19. Gresset-Bourgeois
V, Leventhal PS, Pepin S, Hollingsworth R, Kazek-Duret MP, De Bruijn I, et
al. Quadrivalent inacÂtivated influenza vaccine (VaxigripTetraTM). Expert Rev Vaccines [Internet]. 2018;17:1-11. http://dx.doi.org/10.10 80/14760584.2018.1407650
20. Liu X, Park J, Xia S, et al.
Immunological non-inferiority and safety of a quadrivalent
inactivated influenza vaccine versus two trivalent inactivated influenza
vaccines in China: Results from two studies. Hum Vaccin
Immunother [Internet]. 2022;18:2132798.
http://dx.doi.org/10.1080/216 45515.2022.2132798
21. Varkey
JB, Varkey AB, Varkey B.
Prophylactic vaccinations in chronic obstructive pulmonary disease: current
status. Curr Opin Pulm Med
[Internet]. 2009;15:90-9.
http://dx.doi.org/10.1097/MCP.0b013e3283218356
22. Gentile A, Paget J, Bellei N, Torres JP, et al. Influenza in Latin America: A
report from the Global Influenza InitiaÂtive (GII). Vaccine
[Internet]. 2019;37:2670-8.
http://dx.doi.org/10.1016/j.vaccine.2019.03.081
23. Hirve
S, Newman LP, Paget J, et al. Influenza Seasonality in the Tropics and
Subtropics - When to Vaccinate? PLoS One [Internet]. 2016;11:e0153003.
http://dx.doi.org/10.1371/journal.pone.0153003
24. Centers for Disease Control
and Prevention. CDC Seasonal Flu Vaccine Effectiveness Studies [Internet]. [cited 11 de junio de 2023].
https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
25. Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine
effectiveness in preventing influenza-associated intensive care admissions and
attenuating severe disÂease among adults in New Zealand 2012-2015. Vaccine [Internet]. 2018;36:5916-25.
http://dx.doi.org/10.1016/j.vaccine.2018.07.028
26. Ferdinands
JM, Thompson MG, Blanton L, Spencer S, Grant L, Fry AM. Does influenza
vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research.
Vaccine [Internet]. 2021;39:3678-95.
http://dx.doi.org/10.1016/j.vaccine.2021.05.011
27. Musher DM, Abers MS, Bartlett JG. Evolving
UnderstandÂing of the Causes of Pneumonia in Adults, With Special Attention to
the Role of Pneumococcus. Clin Infect
Dis [Internet]. 2017;65:1736-44.
http://dx.doi.org/10.1093/cid/cix549
28.
DirecciĂłn de Control de Enfermedades Inmunoprevenibles.
VacunaciĂłn contra Neumococo. Lineamientos TĂ©cnicos. Manual del Vacunador.
Ministerio de Salud de Argentina; 2018.
29.
Jiménez Ruiz CA, Buljubasich D, Sansores R, et al. Documento
de consenso SEPAR-ALAT sobre vacunaciĂłn antineumocĂłcica
en fumadores. Arch Bronconeumol [Internet]. 2015;51:350-4.
https://www.sciencedirect.com/science/article/pii/S0300289614004888
30. Jackson LA, Gurtman A, Rice K, Pauksens K, et
al. ImÂmunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in
adults 70 years of age and older previously vaccinated with 23-valent
pneumococcal polysaccharide vaccine. Vaccine [Internet].
2013;31:3585-93.
http://dx.doi.org/10.1016/j.vaccine.2013.05.010
31. Tomczyk
S, Bennett NM, Stoecker C, et al. Use of 13-valent
pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide
vaccine among adults aged ≥65 years: recommendations of the Advisory
Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep [Internet].
2014;63:822-5.
https://www.ncbi.nlm.nih.gov/pubmed/25233284
32. Bonten
MJM, Huijts SM, Bolkenbaas
M, et al. PolysacchaÂride conjugate vaccine against pneumococcal pneumonia in
adults. N Engl J Med [Internet].
2015;372:1114-25.
http://dx.doi.org/10.1056/NEJMoa1408544
33. Dunne EM, Cilloniz
C, von Mollendorf C, et al. PneumoÂcoccal Vaccination
in Adults: What Can We Learn From Observational
Studies That Evaluated PCV13 and PPV23 Effectiveness in the Same Population? Arch Bronconeumol [Internet]. 2023;59:157-64. http://dx.doi.org/10.1016/j.arbres.2022.12.015
34. Vaccines for Pneumococcal
[Internet]. 2023 [cited 2023 Jul 4].
https://www.cdc.gov/vaccines/vpd/pneumo/index.html
35. Essink
B, Sabharwal C, Cannon K, Frenck
R, Lal H, Xu X, et al.
Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and
Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged ≥18
Years. Clin Infect Dis [Internet]. 2022;75:390-8.
http://dx.doi.org/10.1093/cid/ciab990
36.
ComisiĂłn Nacional de Inmunizaciones. Lineamientos tĂ©cÂnicos para la vacunaciĂłn
de personas inmunodeprimidas (huéspedes especiales). Ministerio de Salud de Argentina; 2014.
37. Zheng
C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of
COVID-19 vaccines: a literature review and meta-analysis. Int
J Infect Dis [Internet]. 2022;114:252-60.
http://dx.doi.org/10.1016/j.ijid.2021.11.009
38. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global
impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis [Internet]. 2022;22:1293-302. http://dx.doi.org/10.1016/S1473-3099(22)00320-6
39. Zeng
B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COÂVID-19
vaccines against SARS-CoV-2 variants of concern: a systematic review and
meta-analysis. BMC Med [Internet]. 2022;20:200. http://dx.doi.org/10.1186/s12916-022-02397-y
40. Macchia
A, Ferrante D, Angeleri P,
et al. Evaluation of a COVID-19 Vaccine Campaign and SARS-CoV-2 InfecÂtion and
Mortality Among Adults Aged 60 Years and Older in a Middle-Income Country. JAMA
Netw Open [Internet]. 2021;4:e2130800.
http://dx.doi.org/10.1001/jamanetworkopen.2021.30800
41. Rearte
A, Castelli JM, Rearte R,
et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and
death due to COVID-19 in people older than 60 years in Argentina: a
test-negative, case-control, and retrospective longitudinal study. Lancet [Internet]. 2022;399:1254-64.
http://dx.doi.org/10.1016/S0140-6736(22)00011-3
42. González S, Olszevicki S, Gaiano A, et al.
Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273
vaccines against hospitalisations among children and
adolescents during the Omicron outbreak in Argentina: A retrospective cohort
study. The Lancet Regional Health-Americas [Internet].
2022;13:100316.
https://www.sciencedirect.com/science/article/pii/S2667193X22001338
43. Rotshild
V, Hirsh-Raccah B, Miskin
I, Muszkat M, Matok I.
Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and
network meta-analysis. Sci Rep [Internet]. 2021;11:22777. http://dx.doi.org/10.1038/s41598-021-02321-z
44. Ayoubkhani
D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vacÂcination: community based
cohort study. BMJ
[Internet]. 2022;377:e069676.
http://dx.doi.org/10.1136/bmj-2021-069676
45.
Janssen C, Cachanado M, Ninove L, et al. ImmunogenicÂity
and reactogenicity of heterologous and homologous
mRNA-1273 and BNT162b2 vaccination: A multicenter non-inferiority randomized
trial. EClinicalMedicine [Internet]. 2022;48:101444. http://dx.doi.org/10.1016/j.eclinm.2022.101444
46. Khoury
DS, Cromer D, Reynaldi A, Schlub
TE, Wheatley AK, Juno JA, et al. Neutralizing antibody
levels are highly predictive of immune protection from symptomatic SARS-CoV-2
infection. Nat Med [Internet]. 2021;27:1205-11.
http://dx.doi.org/10.1038/s41591-021-01377-8
47. Bar-On YM, Goldberg Y, Mandel
M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med [Internet]. 2021;385:1393-400. http://dx.doi.org/10.1056/NEJMoa2114255
48. Magen
O, Waxman JG, Makov-Assif M, et al. Fourth Dose of
BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med [Internet]. 2022;386:1603-14.
http://dx.doi.org/10.1056/NEJMoa2201688
49. Wu N, Joyal-Desmarais
K, Ribeiro PA, et al. Long-term effectiveness of
COVID-19 vaccines against infections, hospitalisations,
and mortality in adults: findings from a rapid living systematic evidence
synthesis and meta-analysis up to December, 2022. Lancet
Respir Med [InÂternet]. 2023;11:439-52.
http://dx.doi.org/10.1016/S2213-2600(23)00015-2
50.
Salud actualiza las recomendaciones para la vacunaciĂłn de refuerzo contra
COVID-19 y fomenta su aplicaciĂłn para mantener una protecciĂłn adecuada
[Internet]. Argentina. gob.ar. 2023 [cited 2023 Jun
2].
https://www.argentina.gob.ar/noticias/salud-actualiza-las-recomendaciones-para-la-vacunacion-de-refuerzo-contra-covid-19-y
51. Chalkias
S, Harper C, Vrbicky K, Walsh SR, Essink
B, Brosz A, et al. A Bivalent
Omicron-Containing Booster Vaccine against Covid-19. N
Engl J Med [Internet]. 2022;387:1279-
91. http://dx.doi.org/10.1056/NEJMoa2208343
52. Office of the Commissioner.
Coronavirus (COVID-19) UpÂdate: FDA Authorizes Moderna,
Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as
a Booster Dose [Internet]. U.S. Food and Drug Administration.
FDA; [cited 2023 Jun 9].
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use
53. Collier AY, Miller J, Hachmann NP. Immunogenicity of BA.
5 Bivalent mRNA Vaccine Boosters. New
England Journal of … [Internet]. 2023;
https://www.nejm.org/doi/full/10.1056/NEJMc2213948
54. Link-Gelles
R, Ciesla AA, Fleming-Dutra KE, et al. EfÂfectiveness
of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection -
Increasing CommuÂnity Access to testing program, United States, September-
November 2022. MMWR Morb Mortal Wkly Rep [Internet]. 2022;71:1526-30.
https://www.cdc.gov/mmwr/volumes/71/wr/mm7148e1.htm?s
55. Blankson
JN. Bivalent COVID-19 Vaccines: Can the Original Antigenic Sin Be Forgiven? J
Infect Dis [Internet]. 2023;227:1221-3.
http://dx.doi.org/10.1093/infdis/jiad073
56. Stein C, Nassereldine
H, Sorensen RJ, et al. Past SARS-CoV-2 infection protection against
re-infection: a systematic review and meta-analysis. Lancet
[Internet]. 2023;401:833- 42.
https://doi.org/10.1016/S0140-6736(22)02465-5
57. Kouhpayeh
H, Ansari H. Adverse events following COVÂID-19 vaccination: A systematic
review and meta-analysis. Int Immunopharmacol
[Internet]. 2022;109:108906.
http://dx.doi.org/10.1016/j.intimp.2022.108906
58. Hippisley-Cox
J, Patone M, Mei XW, et al. Risk of thromÂbocytopenia
and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing:
self-controlled case series study. BMJ [Internet]. 2021;374:n1931.
http://dx.doi.org/10.1136/bmj.n1931
59. Kerr S, Joy M, Torabi F, et al. First dose ChAdOx1 and BNT162b2 °COVID-19
vaccinations and cerebral venous sinus thrombosis: A pooled self-controlled
case series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med
[Internet]. 2022;19:e1003927.
https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1003927
60. Cho JY, Kim KH, Lee N, et al.
COVID-19 vaccination-related myocarditis: a Korean nationwide study. Eur Heart J [Internet]. 2023 Jun 2;
http://dx.doi.org/10.1093/eurheartj/ehad339
61. Patone
M, Mei XW, Handunnetthi L, et al. Risks of myoÂcarditis,
pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or
SARS-CoV-2 infection. Nat Med [Internet]. 2022;28:410-22. http://dx.doi.org/10.1038/s41591-021-01630-0
62. Cherry JD. Epidemiological,
clinical, and laboratory aspects of pertussis in adults. Clin Infect Dis [Internet]. 1999;28
Suppl 2:S112-7. http://dx.doi.org/10.1086/515058
63. Edwards KM, Decker MD. Acellular
pertussis vacÂcines for infants. N Engl
J Med [Internet]. Mass MediÂcal Soc. 1996;334:391-2.
http://dx.doi.org/10.1056/NEJM199602083340609
64. Liang JL, Tiwari
T, Moro P, et al. Prevention of Pertussis, Tetanus, and Diphtheria with
Vaccines in the United States: Recommendations of the Advisory Committee on
ImmuniÂzation Practices (ACIP). MMWR Recomm Rep
[Internet]. 2018;67:1-44.
http://dx.doi.org/10.15585/mmwr.rr6702a1
65. Global Strategy for the
Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. 2023 Report. GOLD; 2023. Report
No.: Version 1.3.
66. Hope-Simpson RE. The Nature
of Herpes Zoster: A Long Term Study and a New Hypothesis. Proc R Soc
Med [Internet]. 1965;58:9-20.
https://www.ncbi.nlm.nih.gov/pubmed/14267505
67. Kim JH, DĂaz-Decaro
J, Jiang N, Hwang SJ, Choo EJ, Co M, et al. The adjuvanted recombinant zoster vaccine is efficacious and
safe in Asian adults ≥ 50 years of age: a
sub-cohort analysis of the ZOE-50 and ZOE-70 randomized trials. Hum Vaccin Immunother [Internet].
2021;17:2050- 7. http://dx.doi.org/10.1080/21645515.2020.1859321
68. Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K,
Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent
population in the United States. BMC Infect Dis [Internet]. 2015;15:502. http://dx.doi.org/10.1186/s12879-015-1262-8
69. Pinchinat
S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence
across Europe: results from a systematic literature review. BMC Infect Dis
[Internet]. 2013;13:170.
http://dx.doi.org/10.1186/1471-2334-13-170
70. Harpaz
R, Ortega-Sanchez IR, Seward JF, Advisory ComÂmittee on Immunization Practices
(ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes
zoster: recommendations of the Advisory CommitÂtee on Immunization Practices
(ACIP). MMWR Recomm Rep [Internet]. 2008;57(RR-5):1-30; quiz CE2–4.
https://www.ncbi.nlm.nih.gov/pubmed/18528318
71. Cunningham AL, Lal H, Kovac M, et al. Efficacy
of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med [Internet]. 2016;375:1019-32. http://dx.doi.org/10.1056/NEJMoa1603800
72. Muñoz-Quiles
C, LĂłpez-Lacort M, DĂez-Domingo
J, Orrico- Sánchez A. Herpes zoster risk and burden
of disease in immunocompromised populations: a
population-based study using health system integrated databases, 2009- 2014.
BMC Infect Dis [Internet]. 2020;20:905.
http://dx.doi.org/10.1186/s12879-020-05648-6
73. Heineman TC, Cunningham A, Levin M. Understanding the
immunology of Shingrix, a recombinant glycoprotein E adjuÂvanted herpes zoster vaccine. Curr Opin Immunol
[Internet]. 2019;59:42-8.
http://dx.doi.org/10.1016/j.coi.2019.02.009
74. Gershon
AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding
of the pathogenesis and epidemiology of herpes zoster. J Clin
Virol [Internet]. 2010;48 Suppl 1(Suppl 1):S2-7.
http://dx.doi.org/10.1016/S1386-6532(10)70002-0
75. Sampathkumar
P, Drage LA, Martin DP. Herpes
zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc [Internet]. 2009;84:274-80. http://dx.doi.org/10.1016/S0025-6196(11)61146-4
76.
Kawai K, Gebremeskel BG,
Acosta CJ. Systematic review of incidence and complications of
herpes zoster: towards a global perspective. BMJ Open
[Internet]. 2014;4:e004833.
http://dx.doi.org/10.1136/bmjopen-2014-004833
77. Lal
H, Cunningham AL, Godeaux O, Chlibek
R, DĂez- Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med [Internet]. 2015;372:2087-96. http://dx.doi.org/10.1056/NEJMoa1501184
78. Santibanez
TA, Zimmerman RK, Nowalk MP, Jewell IK, Bardella IJ. Physician attitudes and beliefs associated
with patient pneumococcal polysaccharide vaccination status. Ann Fam Med [Internet]. 2004;2:41-8. http://dx.doi.org/10.1370/afm.53
79. Branche
AR, Saiman L, Walsh EE, Falsey
AR, Jia H, Barrett A, et al. Change in functional
status associated with respiratory syncytial virus infection in hospitalized
older adults. Influenza Other Respi
Viruses [Internet]. 2022;16:1151-60.
http://dx.doi.org/10.1111/irv.13043
80.
DirecciĂłn Nacional de EpidemiologĂa y Análisis de la SituÂaciĂłn de Salud.
BoletĂn Integrado de Vigilancia. Ministerio de Salud de Argentina; 2019. Report No.: 477.
81. CDC. Learn about Respiratory
Syncytial Virus Infection (RSV) [Internet]. Centers for
Disease Control and PrevenÂtion. 2023 [cited 2023 Jun 10].
https://www.cdc.gov/rsv/index.html
82. Papi
A, Ison MG, Langley JM, et al. Respiratory Syncytial
Virus Prefusion F Protein Vaccine in Older Adults. N Engl J Med [Internet]. 2023;388:595-608. http://dx.doi.org/10.1056/NEJMoa2209604
83. Centers for Disease Control
and Prevention (CDC), AdÂvisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal
disease among adults using the 23-valent pneumococcal polysaccharide vaccine
(PPSV23). MMWR Morb Mortal Wkly Rep [Internet]. 2010;59:1102-6.
https://www.ncbi.nlm.nih.gov/pubmed/20814406
84. Centers for Disease and
Prevention. Healthy People 2023 [Internet]. [cited 2023 Jun 9]. https://www.cdc.gov/nchs/healthy_people/hp2030/hp2030.htm
85. Boikos
C, Quach C. Risk of invasive pneumococcal disÂease in
children and adults with asthma: a systematic review. Vaccine
[Internet]. 2013;31:4820-6.
http://dx.doi.org/10.1016/j.vaccine.2013.07.079
86. Vila-CĂłrcoles
A, Ochoa-Gondar O. [Pneumococcal disease in adults: Risk levels and vaccine
recommendations]. Aten
Primaria [Internet]. 2017;49:111–7.
http://dx.doi.org/10.1016/j.aprim.2016.06.007
87.
Sánchez CA. GEMA 5.0: guĂa española para el manejo del asma [Internet]. 2020.
https://books.google.com/books/about/GEMA_5_0.html?hl=&id=emt_zwEACAAJ
88.
Adamuz J, Viasus D,
JimĂ©nez-MartĂnez E, et al. Incidence, timing and
risk factors associated with 1-year mortality after hospitalization for
community-acquired pneumoÂnia. J Infect [Internet]. 2014;68:534-41. http://dx.doi.org/10.1016/j.jinf.2014.02.006
89.
DirecciĂłn Nacional de Control de Enfermedades InmunoÂprevenibles.
Manual del vacunador. Lineamientos TĂ©cniÂcos. Ministerio de Salud; 2022.
90.
Estrategia Nacional de PrevenciĂłn y Control de EnfermeÂdades No Transmisibles.
GuĂa de Práctica ClĂnica Nacional de DiagnĂłstico y Tratamiento de la Enfermedad
Pulmonar Obstructiva CrĂłnica. Ministerio de Salud de Argentina; 2016.
91.
Miravitlles M, Calle M, Molina J, et al.
ActualizaciĂłn 2021 de la GuĂa Española de la EPOC (GesEPOC).
TrataÂmiento farmacolĂłgico de la EPOC estable. Archivos de BronconeumologĂa
[Internet]. 2022;58:69-81.
https://www.sciencedirect.com/science/article/pii/S0300289621001034
92. Kopsaftis Z, Wood-Baker R, Poole P. Influenza vaccine for
chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev [Internet]. 2018;6:CD002733.
http://dx.doi.org/10.1002/14651858.CD002733.pub3
93. Wongsurakiat
P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients
with COPD and the effectiveness of inÂfluenza vaccination: a randomized
controlled study. Chest [Internet]. 2004;1252011-20. http://dx.doi.org/10.1378/chest.125.6.2011
94. MartĂnez-Baz
I, Casado I, Navascués A,
et al. Chronic obÂstructive pulmonary disease and influenza vaccination efÂfect
in preventing outpatient and inpatient influenza cases. Sci
Rep [Internet]. 2022;12:4862.
http://dx.doi.org/10.1038/s41598-022-08952-0
95. Huang CL, Nguyen PA, Kuo PL, Iqbal U, Hsu YHE, Jian WS. Influenza vaccination and
reduction in risk of ischemic heart disease among chronic obstructive pulmonary
elderly. Comput Methods Programs Biomed [Internet].
2013;111:507-11.
http://dx.doi.org/10.1016/j.cmpb.2013.05.006
96. Sehatzadeh
S. Influenza and pneumococcal vaccinations for patients with chronic
obstructive pulmonary disease (COPD): an evidence-based review. Ont Health Technol Assess Ser [Internet]. 2012;12:1-64.
https://www.ncbi.nlm.nih.gov/pubmed/23074431
97. Walters JA, Tang JNQ, Poole
P, Wood-Baker R. PneuÂmococcal vaccines for preventing pneumonia in chronÂic
obstructive pulmonary disease. Cochrane Database Syst
Rev [Internet]. 2017;1:CD001390.
http://dx.doi.org/10.1002/14651858.CD001390.pub4
98. Walters JA, Smith S, Poole P,
Granger RH, Wood-Baker R. Injectable vaccines for preventing pneumococcal
infection in patients with chronic obstructive pulmoÂnary disease. Cochrane
Database Syst Rev [Internet]. 2010;(11):CD001390.
http://dx.doi.org/10.1002/14651858.CD001390.pub3
99. Adler AJ, Eames KTD, Funk S,
Edmunds WJ. Incidence and risk factors for influenza-like-illness in the UK:
online surveillance using Flusurvey. BMC Infect Dis
[Internet]. 2014 May 1;14:232.
http://dx.doi.org/10.1186/1471-2334-14-232
100. Matanock
A, Lee G, Gierke R, Kobayashi M, Leidner
A, Pilishvili T. Use of 13-Valent Pneumococcal
Conjugate VacÂcine and 23-Valent Pneumococcal Polysaccharide Vaccine Among
Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee
on Immunization Practices. MMWR Morb
Mortal Wkly Rep [Internet]. 2019;68:1069- 75. http://dx.doi.org/10.15585/mmwr.mm6846a5
101. Kobayashi M, Farrar JL, Gierke R, Britton A, Childs L, Leidner
AJ, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent
Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of
the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly
Rep [Internet]. 2022;71:109-17.
http://dx.doi.org/10.15585/mmwr.mm7104a1
102. O’Grady KAF, Chang AB, Grimwood K. Vaccines for chilÂdren and adults with chronic
lung disease: efficacy against acute exacerbations. Expert
Rev Respir Med [Internet]. 2014;8:43-55. http://dx.doi.org/10.1586/17476348.2014.8 52960
103. O’Grady KAF, Cripps AW, Grimwood K. Paediatric and adult
bronchiectasis: Vaccination in prevention and manÂagement. Respirology [Internet]. 2019;24:107-14. http://dx.doi.org/10.1111/resp.13446