Autor : Jajati, MĂłnica1, Sivori, MartĂn1, Capelli, Luciano1, Pascansky, Daniel1, Catania, Iván2, González, Laura3, Mancuso, Marcela3
1 Pulmonology and Tisiology Unit. Pulmonology University Center, University of Buenos Aires (UBA). Hospital “Dr. J. M. Ramos Mejia.” Autonomous City of Buenos Aires. Argentina. 2Tuberculosis Program of the Ministry of Health of the Government of the Autonomous City of Buenos Aires. 3Billing Department. Hospital “Dr. J. M. Ramos Mejia.” Autonomous City of Buenos Aires. Argentina.
https://doi.org/10.56538/ramr.HRPD4589
Correspondencia : MĂłnica Jajati. Urquiza 609. CP 1405. Correo electrĂłnico: mjajati@yahoo.com.ar
RESUMEN
Objetivo:
Determinar
costos directos del tratamiento en tres grupos de pacientes con tuberculosis
pulmonar (TP): ambulatorios-adherentes (AA), hospitalizados adherentes (HA) y
hospitalizados no adherentes (HNA).
Materiales
y métodos: Se
consideraron tres grupos: AA, HA y HNA. Se determinaron costos directos desde
la perspectiva del financiador, segĂşn modulaciĂłn del Gobierno de la Ciudad de
Buenos Aires (GCBA) a julio 2022, cotizaciĂłn peso/dĂłlar 140. El costo de las
drogas antituberculosis fue provisto por el Programa
de Tuberculosis del GCBA.
Resultados:
Se
incluyeron 10 pacientes AA, tiempo de tratamiento 24±2.52 semanas, adherencia
100 %. El costo directo fue 257.79 dĂłlares/paciente (RIQ=191.6-328.55). Se
incluyeron 20 pacientes HNA y 10 HA, sin diferencias en edad y género entre
ellos. Los HNA tenĂa mayor carga tabáquica, situaciĂłn de calle, desnutriciĂłn,
alcoholismo, adicÂciones y HIV (todos p<0.05). El tiempo de primer
tratamiento fue para HNA 5.5 semanas (RIQ=3-8) y 24 semanas para HA. La
duraciĂłn en HNA de siguientes tratamientos fue 0.5-9 semanas. El costo final
fue 8165.87 dĂłlares/paciente (RIQ=4706.45-12 897.82) en HNA y 4015.26 dĂłlares
(RIQ=3458.15-4482.6) en HA (p<0.01).
ConclusiĂłn:
El
costo directo del tratamiento en AA fue 257 dĂłlares/paciente. El costo directo
del abandono del tratamiento de HNA es el doble que HA (8165 vs. 4015 dĂłlares).
El costo de tratar a AA es quince veces menor que internarlos. Es el primer
estudio de costos directos en nuestro paĂs sobre el tema. Se deben instrumentar
programas de mejora de adherencia al tratamiento para evitar alto costo
sanitario, drogo-resistencia y aumento de la morbi-mortalidad.
Palabras
clave: Tuberculosis
pulmonar;, HospitalizaciĂłn, Tratamiento, Adherencia,Abandono
Received: 10/22/2023
Accepted: 01/24/2024
INTRODUCTION
Tuberculosis (TB) remains an
unresolved global health problem, particularly affecting poor, deÂveloping
countries; and it can be associated with other comorbidities or not. There is a
significant percentage of underdiagnosis and
treatment withdrawal due to a lack of education and socioÂeconomic factors.1
The World Health Organization
(WHO) report of 2022 revealed that 6.4 million people contracted TB in 2021, a
figure that underestimates the true impact of the disease due to underreporting
linked to the pandemic.2 It is
estimated that there were 1.4 million deaths in individuals without human
immunodeficiency virus (HIV) infection and 187,000 deaths among people with
HIV.2
In 2021, the Ministry of Health
of Argentina reported 12,569 TB cases, representing a TB noÂtification rate of
27.4/100,000 inhabitants (13.5 % higher than in 2020 and slightly lower than in
2019 at 28.2, used as a reference so as not to consider the pandemic effect).
The cases were concentrated at 59.8 % in individuals aged 15 to 44, with a
mortality rate of 1.49/100,000: 6 % higher than in 2018 (26.2/100,000).3 The
Autonomous City of Buenos Aires (CABA) together with the province of Buenos
Aires had the highest number of cases, with 65.67 % of the country’s reported
cases (39.8 and 39.7 per 100,000 inhabitants, respectively).3
Withdrawal of antituberculous
treatment is a significant obstacle to disease control. The conseÂquences of
non-adherence to treatment include the continuation of the disease transmission
chain, the development of bacterial resistance mechanisms, a subsequent
increase in morbidity and mortality, and an escalation of treatment costs with
an imÂpact on healthcare services. Various studies have investigated the cost
of TB treatment, both direct and indirect, in many countries worldwide, but to
our knowledge, there are no such investigations in our country.4-16
The expenditure on TB treatment
is half the cost of human immunodeficiency virus treatment and less than a
tenth of the cost of COVID-19 treatment. This spending has been decreasing year
by year, despite tuberculosis being the infecÂtious disease with the highest
mortality until the year 2020.17
The objective of this study is to
determine the direct costs, from the perspective of the funder of the treatment
of patients with pulmonary TB, including adherent outpatients (AOs) and hospiÂtalized
individuals, comparing adherent (HA) with non-adherent (HNA) patients. This
research is conducted within the setting of a multispecialty public hospital in
CABA.
MATERIALS AND METHODS
The medical records of patients
diagnosed with pulmonary TB upon admission to our hospital between the years
2017 and 2021 were retrospectively evaluated. Direct costs were determined from
the perspective of the funder, taking into account medication costs and the
hospitalization cost modules of the Government of the City of Buenos Aires
(GCBA) as of July 2022, at an exchange rate of 140 pesos/ dollar (Banco NaciĂłn Rep. Argentina).
Argentina). The cost of antituberculous drugs was
provided by the TB Program of the Ministry of Health of the GCBA. The cost of
drugs outside the cost modules was determined using the pharÂmaceutical manual Kairos of July 2022 and the diagnostic studies of
nomenclature guidelines of the GCBA.
Three groups of patients were
considered for cost analyÂsis: adherent outpatients (AOs),
hospitalized-adherent (HA), and hospitalized non-adherent (HNA). To select the
patient profile to be included in the AOs group, a sample was taken
representing in its demographic, clinical, and social characteristics the
profile of the entire database of patients treated on an outpatient basis in
our Unit. A number of hospitalized patients were included for the analysis in a
2:1 ratio for the HNA:HA groups. A patient was
considered non-adherent if they discontinued the antiÂtuberculous
treatment for more than 4 weeks and without any medical supervision.1 For hospitalized patients who were non-adherent from the second
admission onwards, sputum GeneXpert and solid culture
tests were requested for each admission. Central (median) and dispersion (IQR
25-75 %) measures were used for quantitative variables, and percentages were
used for categorical variables. For the comparison between subgroups HNA and HA
of categorical variables, the Fisher’s test was used. The statistical package
of the Biostat program was used.
RESULTS
The demographic characteristics
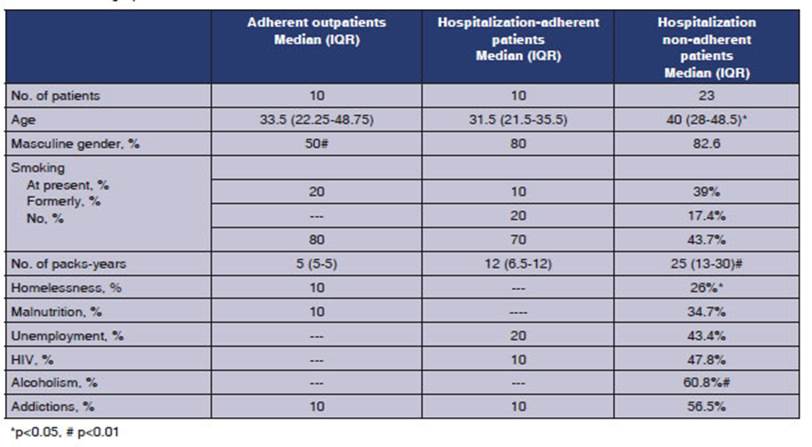
of the 3 groups are detailed in Table 1.
Cost of adherent outpatient group
10 patients were included. The
average treatment duration was 24 weeks, with 100 % adherence to the treatment
regimen of 2 months of isoniazid-riÂfampicin-pyrazinamide-ethambutol
and 4 months of isoniazid-rifampicin (2HRZE/4HR). The median total direct costs
per patient were US$ 257.79 (IQR=191.6-328.55).
Cost of hospitalized-adherent group
10 patients were
included. One patient died. One patient was found to have rifampicin resistance
durÂing follow-up. The average treatment duration was 24 weeks, with 100 %
adherence to the 2HRZE/4HR treatment regimen. The total direct cost per patient
was US$4,015.26 (IQR= 3,458.15- 4,482.6).
Cost of hospitalized non-adherent group
20 patients were
included. The first treatment began with HRZE, and those who could move on to
the second phase were treated with HR. The duraÂtion of the first treatment was
6 weeks (IQR=3-9). The duration of the second to the sixth treatment ranged
from 0.5 to 9 weeks. Fourteen patients underwent two treatments (duration of 8
weeks, IQR=4.5-12); eight patients underwent three treatments (duration of 6
weeks, IQR=5-12); two patients underwent four treatments (duration of 4 weeks),
and one patient underwent six treatments (0.5 week). In 100 % of treatment
withdrawal cases, the patient left the hospital voluntarily. Only one patient
died (human immunodeficiency virus-HIV) one year after the second hospitalizaÂtion
due to an undiagnosed space-occupying mass. The number of visits to the
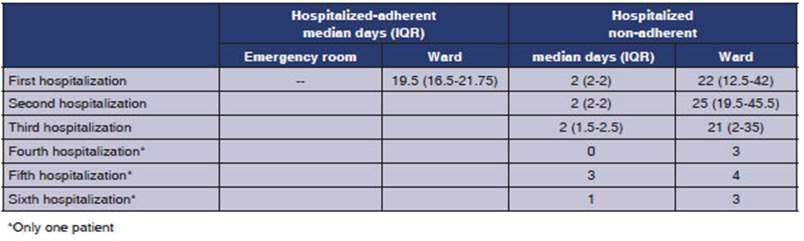
emergency room, the inpatient ward, and the Intensive Care Unit are detailed in
Table 2. The total direct cost per patient was US$8,165.87 (IQR=4,706.45-12
897.82).
Comparison between hospitalized-adherent and
non-adherent groups
Table 1 shows the
demographic variables of both groups. There were differences in age between the
groups (older age in the HNA group, p<0.05). Regarding the gender, there was
a predominance of males among hospitalized patients (p<0.05). In the HNA
group, there were more patients with smoking load, homelessness (p<0.05),
unemployÂment, malnutrition, alcoholism, drug addiction, and reactive HIV
serology (for the rest p<0.01). There were no differences during the first
hosÂpitalization between both groups (HA: 21.5 vs. HNA: 19 days).
When comparing the
final cost per patient of both hospitalized patient groups, a significant
difference was observed (p<0.01). Comparing the direct non-modular costs of
both hospitalized paÂtient groups, a significant difference was observed (33 %
for the HNA group vs. 8.29 % for the HA group, p<0.05).
DISCUSSION
The direct cost of
complying with pulmonary TB outpatient treatment in a public hospital of the
city of Buenos Aires is US$257 per patient. The patient profile could be
representative of those treated in the public healthcare system. The direct
cost of treatment withdrawal per hospitalized patient who began treatment for
pulmonary TB is twice the cost of patients who complete the treatment (US$8,165
vs. US$ 4,015). In the HNA group, there were more patients with smoking load,
homelessness, unemployment, malnutrition, alcoÂholism, drug addiction, and
reactive HIV serology (p<0.05). The cost of treating outpatients is fifteen
times lower than the cost of hospitalizing them.
The WHO “End TB” strategy for the
year 2025 includes reducing the incidence rate by 50 % in the 2015-2025 decade,
reducing mortality by 75 %, and bringing the
percentage of patients with cataÂstrophic costs to 0.2 As of 2021, these three
indicaÂtors were at 10 %, 5.9 %, and 48 %, respectively, far from the target to
be achieved.2 Moreover, an important related issue is the worryingly
low rate of treatment adherence. The WHO defined adherÂence in 2003 as “the
degree to which a patient’s behavior, in terms of medicine-taking, following a
diet, or making lifestyle changes, corresponds with the recommendations of the
healthcare provider.”18 There are tools for the assessment of
adherence, such as the Morinsky Green questionÂnaire.19
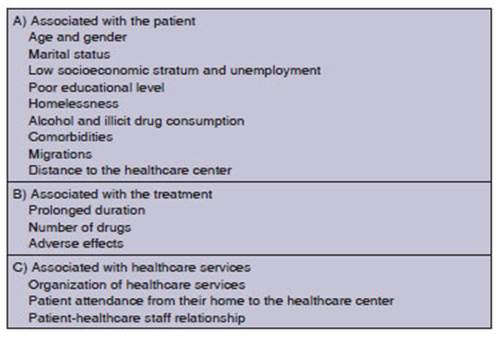
Factors related to the adherence to antiÂtuberculous
treatment are detailed in Table 3.20-25 Among
those factors associated with the patient, young men in economically active
ages are less adherent than women. This has been observed in various studies,
including our study. Single men are more likely to withdraw treatment compared
to married individuals, probably due to lack of family support. The low
socioeconomic status and unemployment lead to precarious living conditions, as
shown in our study. Also, low schooling rates are associated with lower
treatment adherence. In some studies, non-compliance was five times greater in
illiterate patients compared to those with primary or secondary education.20
Our study includes other factors that are related to higher withdrawal rates:
homelessness, lack of housing, alcoholism, and addiction to illicit drugs.21-24
Also the number and type of comorbidities, such as HIV, psychiatric disorders,
physical impairment, etc. The migration of individuals from
one counÂtry to another after starting treatment also make compliance
difficult. In Argentina, Herrero et al conducted a
study in the Buenos Aires MetropoliÂtan Area on 123 TB patients (38
non-adherent and 85 adherent) to identify factors associated with
non-adherence.25 They determined that the factors mostly associated
with non-adherence were: male gender (Odds Ratio, OR=2.8), patients attending a
hospital (OR=3.4), and those facing difficulties due to transportation costs
(OR=2.5).25 In the 2022 Tuberculosis Epidemiological Bulletin of
Argentina, out of 12,569 cases reported in 2021, 31 % lacked treatment
evaluation records, which may imply possible treatment withdrawal in many of
those cases.3 Among the factors associated with the treatment,
prolonged duration is one of the main patient complaints. Patients also
complain about the number of drugs. The number of tablets affects adherence,
and this factor worsens if the paÂtient has other comorbidities requiring
additional concomitant medications (e.g., antiretrovirals).
Finally, adverse events such as diarrhea, vomiting, allergic reactions, liver
disease, ototoxicity, etc.20 Among the
factors associated with health services, organization is crucial, with health
staff training, including specialized doctors being essential for the
implementation of an adequate TB program. Also, the distance from the patient’s
home to the health center is an important factor that should be considered:
greater distance usually correlates with lower adherence, as confirmed by
various studies.20,25 Lastly, the healthcare provider-patient
relationship is crucial, both with the doctor and the person administering the
medication, to ensure the patient feels supported.
The consequences of
non-adherence to treatÂment can be evaluated from both the patient’s and the
society’s standpoint. For the patient, it can lead to the worsening of the TB
clinically, potentially resulting in death, or the development of antibiotic resistance,
thus causing personal and social conseÂquences. Consequently, future treatments
will not yield the expected response. Multidrug-resistant TB (MDR-TB) is a
growing global problem related to non-adherence, among other factors.1-3
Social consequences are related to the emergence of MDR-TB but also to the
economic consequences of increased consumption of health resources.26-27
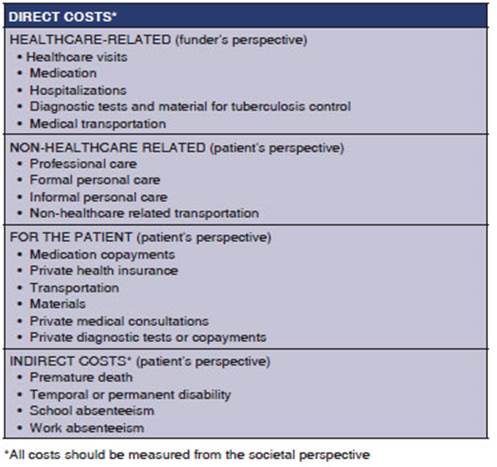
Economic consequences can be analyzed from the perspective of the funder, the
patient, or the society as a whole. This includes direct costs (medical:
expenses for the maintenance of health centers and equipment amortization,
healthcare team fees, diagnostic tests, and treatments; non-medical:
transportation, meals) and indirect costs (loss of business days, decreased
productivity, and ecoÂnomic loss due to premature death). However, we should
also highlight the fact that when compariÂsons between studies are attempted,
costs depend on the healthcare structure, which is inherent to each country,
and financing system. Therefore, it’s not advisable to make such comparisons
(Table 4).
In reviews regarding
the economic consequences of TB treatment, 71 studies were identified for
drug-susceptible TB, 10 for MDR-TB, and nine for both.26-27 These studies were conducted in 50 and 16 countries,
respectively. They were conducted from the perspective of the funder (31 %),
the paÂtient (26 %), and both (43 %).26 From the funder’s
perspective, the cost of drug-susceptible TB was US$14,659 in high-income
countries, US$840 in middle-income countries, US$273 in low to middle-income
countries, and US$258 in low-income countries.26-27 The treatment
cost for MDR-TB was US$83,365, US$5,284, US$6,313, and US$1,218,
respectively.26-27 From the patient’s perspective in drug-susceptible TB, an
additional cost of 3 % was observed in high-income countries, 72 % in
middle-income countries, 60 % in low-to-middle-income countries, and 31 % in
low-income countries.26-27 When combining all the costs,
productivity loss was 16 %, 2 %, 40 %, and 38 %, respectively.27
In a study by the
WHO’s Global TB Program, Tanimura et al determined an
average cost of US$847 per patient (20 % direct medical costs, 20 % direct
non-medical costs, and 60 % indirect costs [income loss]), 50 % before TB
treatment.28
Among developed
countries, there are few studÂies evaluating the cost impact on the healthcare
system.4-10,29 Chan et al in Australia determined a treatment cost of
11,538 Australian dollars for ten patients with drug-susceptible pulmonary TB.4
Only one patient with MDR-TB was included in the cost comparison analysis
(258,089 Australian dollars, that is to say, twenty-two times more).4
Breaking down the costs, diagnosis accounted for 12 %, hospitalization 43 %,
outpatient conÂsultations 5 %, medication 7 %, and community programs and other
costs 26 %. Oh et al reviewed eighteen studies on costs in the United States,
findÂing an average direct cost per patient of US$34,600 for drug-susceptible
TB and US$110,900 for MDR-TB (three times more).5 Breaking down the
costs, outpatient consultations were US$4,300 per patient, laboratory tests
averaged US$1,500 per patient, and antibiotics were $800 per patient (2 % for
drug-susceptible TB patients and 27 % for MDR-TB patients).5 The
cost of hospitalized TB in the United States was US$34,512, and for outpatient
care, US$3,296 .6-7 The indirect costs due to productivity loss
during hospitalization were US$404 and US$403 per patient, respectively, for
the outpatient period.7 Grosse et al estimated the social indirect
cost of premature death to be US$574,751 per patient at the age of 65.8 Marks
et al estimated the costs for MDR-TB and extenÂsively drug-resistant TB9 in the
United States. The outpatient cost was US$83,909 and US$221,916 per patient in
2020, respectively. For hospitalized patients, it was US$98,278 and US$345,792
per patient in 2020, respectively. Productivity loss was estimated at
US$165,137 per patient in 2020 for MDR-TB patients. For extensively
drug-resistant TB patients, productivity loss was US$161,331 per patient in
2020. The cost of premature death at the age of 55 was calculated at
US$1,031,513 per patient in 2020.9 In Europe, Diel
et al reviewed TB management costs in the 27 countries of the European
Community.10 Taking into account diÂrect and indirect costs, the
total cost was €10,282 for drug-susceptible TB, €57,213 for MDR-TB and €170,744
for extensively drug-resistant TB.10 Law et al developed a Markov theoretical
model for cost-effectiveness to evaluate four different treatment regimens for
MDR-TB with varying prevalence percentages.29 Reinforced retreatment
regimens were the most cost-effective schemes. However, the initial empirical
regimen for MDR-TB, despite being more expensive, results in lower mortality
compared to the traditional scheme, with an average cost of US$4,650 per
patient per every day with good-quality of life obtained.29 In most
middle-income countries, this is below the per capita income, which is the
usual threshold taken as the incremental cost-effectiveness ratio (ICER).29
Among the developing
countries, there are two Latin American studies.11-12 Steffen et al
from BraÂzil described the cost of directly observed therapy (DOT) versus
self-administered treatment in 218 patients with pulmonary TB from the
patient’s and the healthcare system perspectives.11 The cost during
the pre-diagnostic phase was higher for the self-administered treatment,
whereas during the treatment phase, it was higher for the DOT. The cost of
complete self-administered treatment was US$194 for the patient and US$189 for
the funder. For DOT, it was US$336 and US$726 respectively, nearly twice the
cost of self-administered treatÂment, with completion rates of 79 % and 71 %
respectively.11 In Ecuador, Rouzier et al
reported from a group of 104 patients with susceptible TB a total cost of
US$960 per patient from the patient’s perspective, and for 14 patients with
MDR-TB, the cost was US$6,880 per patient (six times higher).12 This
represents 31 % and 223 % of Ecuador’s averÂage per capita income. In patients
with MDR-TB, the cost was higher due to loss of income.12 Among
Asian countries, Chandra et al reviewed 13 studies on
TB treatment costs in India.13 The cost from the patient’s
perspective was US$235, with 45.5 % being direct costs in the public system.
Only one study in the private system for drug-resistant TB determined a total
direct cost of US$7,778.13 In Indonesia, McAllister et al determined
the treatÂment cost from the patient’s perspective in 106 TB patients. The
average cost was US$243.66 per patient.14 In 2011, Long et al
conducted a systemÂatic analysis of the direct cost of antituberculous
treatment in relation to adherence in China.15 While basic diagnosis
(X-ray and bacilloscopy) and antibiotic treatment are
free, a high percentage of withdrawal (73 %) was observed associated with the
transportation and medical fees that patients have to pay. In the Chinese
review, the outpatient cost from the patient’s perspective ranged from US$149
to US$724, representing between 42 % and 119 % of household income and
considered one of the main factors. It should be noted that one-third of the
world’s MDR-TB patients are from China, and this can explain the high withdrawal
rate, among other factors.15 More recently,
in 2020, Xu et al reported a study on 326 TB
patients, with 21.4 % below the poverty line.16 The total treatment
cost per patient was US$1,185.5 (88 % direct costs). Of this total, 37 %
occurred before TB care. Factors associated with higher costs were elderly age,
being divorced or living alone, residÂing in rural areas, greater poverty, and
incomplete primary education.
The WHO defines
catastrophic costs for TB as the direct and indirect costs of treatment that exÂceed
20 % of a person’s annual economic income. In a systematic analysis, 29 studies
were selected out of 5,114 studies.30 The
average proportion of catastrophic costs was 43 %. The major predictors of high
catastrophic cost were country variables, MDR-TB, and HIV. Catastrophic costs
were lower in active case-finding strategies (12 %) compared to passive waiting
strategies (30 %).30 In a study by the TB Program of the WHO, Tanimura et al determined that catastrophic costs accounted
for 58 % of individual annual income, especially among the poorest people with
MDR-TB.28 GuiÂdoni et al evaluated the
cost of 350 TB patients prospectively in five Brazilian cities.31
UnfavorÂable outcomes were associated with catastrophic costs (OR=2.53, 95
%CI=1.13-5.67) and divorce (OR=5.29, 95 %CI=1.3-20.05).31 In India,
Chandra et al conducted a review of 13 studies on costs and determined that
catastrophic cost ranged from 7 to 32.4 % in patients with drug-susceptible TB
and 68 % in drug-resistant TB patients.13 In IndoneÂsia, McAllister
et al found that 26.5 % of patients exceeded 20 % of their annual income.14
Few studies were
found combining analyses and relating the implications of poor adherence to TB treatment
with the impact on healthcare costs. For purposes of comparing costs, Kwon et
al reported on 3,799 TB patients, 2,662 adherent and
1,137 non-adherents.32 Five years later, the costs for adherent
patients were US$2,270, and US$2,694 for non-adherent patients. The monthly
cost was 11 % lower for adherent patients, and the total cost for non-adherent
patients was two and a half times higher.32 Chimeh
et al conducted a systematic analysis between 2009 and 2019 where they compared
the costs of TB management with non-adherence.33 Out of 14 studies,
8 focused on non-adherence and death, 2 on treatment failure, 1 on treatment
success, 1 on treatment successes and failures, and 2 on costs. Most studies
were retrospective or case-control studies.33 The
results showed that non-adherence was associated with higher mortality,
treatment failure, lower cure rates, and a significant negative economic
impact.33
In 2011, Long et al
conducted a systematic analÂysis of the direct cost of antituberculous
treatment in relation to adherence in China. Despite the fact that antibiotic
treatment is free, a high percentage of withdrawal (73 %) was observed, which
was asÂsociated with the amount of money the patient has to pay, for example in
transportation and medical fees.15 In our study, the direct cost of
treating outÂpatients is fifteen times lower than hospitalizing them, and the
cost of withdrawing treatment in the case of a hospitalized patient with
pulmonary TB is twice the cost of an adherent patient (US$8,165 vs. US$ 4,015
per patient, respectively).15 Factors associated with non-adherence
included higher smoking load, homelessness, malnutrition, unÂemployment,
alcoholism, substance abuse, and reactive HIV serology.15
Among the limitations
of this study, it can be said that data collection from medical records was retÂrospective.
Another limitation is that extrapolating its conclusions to other healthcare
systems in our country or other regions (external validity) is not advisable
due to the previously mentioned differing cost structures. Another limitation
is that indirect costs were not evaluated (which are presumed to be higher than
direct costs based on previously reviewed literature); and costs were not
determined from other perspectives (for example, patient or societal perspecÂtives).
While costs were initially calculated in pesos, the currency instability and
devaluation experienced by our country in recent times led us to report the
results in dollars (taking into account the exchange rate of the beginning of the
study).
Also, the fact that
there is a small number of patients in the study is a limitation, thus, concluÂsions
drawn from the statistical significance found should be evaluated with
discretion.
Finally, another
limitation would be that the cost modules used by the GCBA did not allow
breaking down the internal cost structure to deÂtermine which variables have
been considered and to what extent.
In conclusion, the
direct cost of complying with outpatient treatment of pulmonary TB in a public
hospital of the city of Buenos Aires is US$257 per patient. The patient profile could be representative of those treated in
the public healthcare system. This is the first study in our country related to
the direct costs of outpatient treatment of pulmonary TB in adherent patients.
The cost of treating outpatients is fifteen times lower than the cost of
hospitalizing them. The direct cost of treatment withdrawal per hospitalized
patient who begins treatment for pulmonary TB is twice the cost of patients who
complete the treatment (US$8,165 vs. US$ 4,015). In the HNA group, there were
more patients with smoking load, homelessness, unemployment, malnutrition,
alcoholism, drug addiction, and statistically significant reactive HIV
serology. It is essential to achieve treatment adherÂence in order to cure TB
and avoid drug resistance problems due to its social and health impact and
increased morbidity and mortality. Interventions should be implemented to
improve adherence, such as providing economic incentives to patients and
educating them on their disease. Healthcare personnel, on the other hand,
should prioritize the care of TB patients, minimize waiting times, and improve
their relationship with the patient.
Conflict of interest
Authors have no conflict of
interest to declare.
REFERENCES
1.
Abbate E, Ballester D, Barrera L, et al. Consenso
Argentino de Tuberculosis. Rev Am Med
Resp. 2009;9:61-99.
2. World Health Organization. Global Tuberculosis Report 2022. Geneva: WHO; 2022.
Acceso el 5 de septiembre de 2022 en https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
3.
BoletĂn sobre Tuberculosis y Lepra en la Argentina N° 6. Año VI-Marzo 2023.
Ministerio de Salud de la NaciĂłn. Acceso el 5 de septiembre de 2023 en https://bancos.salud.gob.ar/sites/default/files/2023-03/Boletin_Tuberculosis_y_Lepra_en_la_Argentina_2023.pdf
4. Chan EC, Nolan A, Denholm JT. How much does tubercuÂlosis cost? An Australian healthcare perspective analysis. CDI. 2017;41: E191-4.
5. Oh P, Pascopella
L, Barry P, Flood JM. A systematic syntheÂsis of direct costs to treat and
manage tuberculosis disease applied to California, 2015. BMC
Res Notes. 2017;10:434.
https://doi.org/10.1186/s13104-017-2754-y
6. Aslam
MV, Owusu-Edusei K, Marks SM, et al. Number and cost
of hospitalizations with principal and secondary diagnoses of tuberculosis,
United States. Int J Tuberc
Lung Dis. 2018;22:1495-504.
https://doi.org/10.5588/ijtld.18.0260
7. Shepardson
D, Marks SM, Chesson H, et al. Cost-effectiveÂness of
a 12-dose regimen for treating latent tuberculous
infection in the United States. Int J Tuberc Lung Dis. 2013;17:1531-7.
https://doi.org/10.5588/ijtld.13.0423
8. Grosse SD, Krueger KV, Pike J.
Estimated annual and lifeÂtime labor productivity in the United States, 2016:
implicaÂtions for economic evaluations. J Med Econ.
2019;22:501-8.
https://doi.org/10.1080/13696998.2018.1542520
9. Marks SM, Flood J, Seaworth B, et al; TB Epidemiologic Studies Consortium. Treatment practices, outcomes, and costs of multidrug-resistant and
extensively drug-resistant tuberculosis, United States, 2005-2007. Emerg Infect Dis. 2014;20:812-21.
https://doi.org/10.3201/eid2005.131037
10. Diel
R, Vandeputte J, de Vries
G, Stillo J, Wanlin M, NienÂhaus A. Costs of tuberculosis disease in the European
Union: a systematic analysis and cost calculation. Eur
Respir J. 2014;43:554-65.
https://doi.org/10.1183/09031936.00079413
11. Steffen R, Menzies D, Oxlade O, et al. Patients´s costs and cost-effectiveness of tuberculosis
treatment in DOTS and Non-DOTS facilities in Rio Janeiro, Brazil. PLos ONE. 2010;5: e14014. https://doi.org/10.1371/journal.pone.0014014
12.
Rouzier VA, Oxlade O, Verduga R, et al. Patient and
family costs associated with tuberculosis, including multidrugreÂsistant
tuberculosis, in Ecuador. Int J Tuberc
Lung Dis. 2010;14:1316–22.
13. Chandra A, Kumar R, ant S, Parthasarathy R, Krishnan A. Direct and indirect patients
costs of tuberculosis in India: Trop Med Inter Health 2020;25:803-12.
https://doi.org/10.1111/tmi.13402
14. McAllister SM, Lestari BW,
Sullivan T, et al. out-of-pocket costs for patients with tuberculosis in
different healthcare settings in Bandung, Indonesia. Am J Trop Med Hyg. 2020;103:1057-64. https://doi.org/10.4269/ajtmh.19-0848
15. Long Q, Smith H, Zhang T,
Tang S, Garner P. Patient mediÂcal costs for tuberculosis treatment and impact
on adherÂence in China: a systematic review. BMC Public
Health. 2011;11:393.
https://doi.org/10.1186/1471-2458-11-393
16. Xu C,
Xia Y, Hu D, Zhang X, Zhao Y. Financial burden of tuberculosis patients: China
2020; China CDC. 2021;5:266- 70.
17. Global Burden of Disease
Health Financing CollaboraÂtor Network. Health sector spending and spending on
HIV/AIDS, tuberculosis, and malaria, and development assistance for health:
progress towards Sustainable DeÂvelopment Goal 3. Lancet 2020; 96:693–724. 6
Stop TB Partnership. The global plan
to end TB 2018–2022. 2019.
http://www.stoptb.org/global/plan/plan1822.asp (accessed July 7, 2021).
18. Adherence to long term
therapies: evidence for action. Geneva: World Health Organization; 2003.
19. Morisky
DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported
measure of medicaÂtion adherence. Med Care.
1986;24:67-74. https://doi.org/10.1097/00005650-198601000-00007
20.
Manjarrez Morales, Evelyn M, Serrano Montes V, et al. Principales causas de
abandono del tratamiento contra la tuberculosis pulmonar. Gac
Med Mex. 1993;129: 57-62.
21.
Albuquerque M, Ximenes R, Lucena-Silva N, et al. Factors associated with treatment failure, dropout, and death in a
cohort of tuberculosis patients in Recife, Pernambuco
State, Brazil. Cad Saude Publica. 2007;23:1573-82.
https://doi.org/10.1590/S0102-311X2007000700008
22. Przybylski
G, Dąbrowska A, Trzcińska
H. Alcoholism and other socio-demographic risk factors for adverse TB-drug
reactions and unsuccess full tuberculosis treatment
-data from ten years' observation at the Regional Centre of Pulmonology,
Bydgoszcz, Poland. Med Sci Monit. 2014;20:444-53.
https://doi.org/10.12659/MSM.890012
23. Naing
NN, DÉste C, Isa AR, Salleh
R, Bakar N, Mahmod MR.
Factors contributing to poor compliance with anti-TB treatment among
tuberculosis patients. South Asian J Trop Med Public Health.
2001;32:369-82.
24. Dilla
T, Valladares A, Lizán L, Sacristán JA. Adherencia y persistencia terapeútica:
causas, consecuencias y estrateÂgias de mejora. Aten Primaria. 200;41:342-8.
https://doi.org/10.1016/j.aprim.2008.09.031
25. Herrero
MB, Ramos S, Arrossi S. Determinants of non-adherence
to tuberculosis treatment in Argentina: barriers related to access to
treatment. Rev Bras Epidemiol 2015; 18:287-98.
https://doi.org/10.1590/1980-5497201500020001
26. Cox H, Furin
J. The incalculable costs of tuberculosis. Lancet Respir. 2021;9: e1337-8. https://doi.org/10.1016/S2214-109X(21)00345-4
27. Laurence YV, Griffiths UK, Vassali A. Costs to health serÂvices and the patient of
treating tuberculosis: a systematic literature review. Pharmacoeconomics. 2015;33:939-55. https://doi.org/10.1007/s40273-015-0279-6
28. Tanimura,
T. et al. Financial burden for tuberculosis patients in low-and middle-income
countries: A systemÂatic review. Eur Respir J 2014;43:1763–75.
https://doi.org/10.1183/09031936.00193413
29. Law S, Benedetti A, Oxlade O, Schwartzman K, Menzies
D. Comparing cost-efectiveness of standardized
tuberculosis treatments given varying drug resistance. Eur
Respir J 2014;43:566-81.
https://doi.org/10.1183/09031936.00005613
30. Ghazy
RM, El Saeh HM, Abdulaziz
S, et al. A systematic review and meta-analysis of the
catastrophic costs incurred by tuberculosis patients. Nature. 2022; 12:558.
https://doi.org/10.1038/s41598-021-04345-x
31.
Guidoni LM, Zandonade E,
Fregona G, et al. CataÂstrophic
costs and social sequels due to tuberculosis diagnosis and treatment in Brazil. Epidemio Serv Saude. 2021;30:e2020810.
32. Kwon SH, Nam JH, Kim SL, Park
HY, Kwon JW. Real-world association of adherence with outcomes and economic
burden in patients with tuberculosis from Sout Korea
claims data. Front Pharmacol. 2022;13:918344.
https://doi.org/10.3389/fphar.2022.918344
33. Chimeh
RA, Gafar F, Pradipta IS,
et al. Clinical and ecoÂnomic impact of medication non-adherence in drug susÂceptible
tuberculosis: a systematic review. Int J Tub Lung Dis.
2020; 24:811-9. https://doi.org/10.5588/ijtld.19.0754