Autor :Arrojo, Marisol1, González, Alejandra1, Zurita, Ingrid1, Fielli, Mariano1, Carreño, Ruth1
1Pulmonology Service. Hospital Nacional Prof. Dr. Alejandro Posadas. El Palomar. Province of Buenos Aires. Argentina.
https://doi.org/10.56538/ramr.ANCR4652
Correspondencia : Marisol Arrojo marisolarrojo@hotmail.com
ABSTRACT
We
present the case of a patient diagnosed with metastatic lung adenocarcinoma
who, after five months of treatment with pembrolizumab, presented grade 2
pneumonitis, interÂpreted as pembrolizumab toxicity, with a good response and
resolution of the infiltrates with the suspension of the immunomodulator and
the administration of corticosteroids.
Key
words: Pembrolizumab;
Pulmonary Toxicity; PD-L1 Positive; non-small cell lung cancer (NSCLC)
RESUMEN
Se
presenta el caso de un paciente con diagnóstico de adenocarcinoma de
pulmón metastásico que, luego de realizar cinco meses de
tratamiento con pembrolizumab, presentó neumonitis grado 2, interpretada
como toxicidad por pembrolizumab con buena respuesta y resolución de los
infiltrados con la suspensión del inmunomodulador y la
administración de corticoides.
Palabras
clave: Pembrolizumab;
toxicidad pulmonar; PD-L1 positivo; cáncer de pulmón de
células no pequeñas (CPNCP)
Received: 11/08/2022
Accepted: 03/23/2023
INTRODUCTION
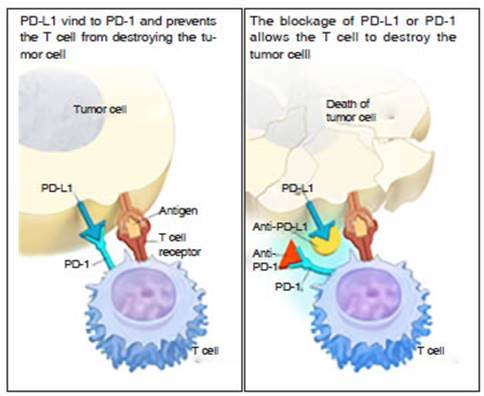
Pembrolizumab
is a monoclonal antibody desigÂnated for the treatment of patients with
metastatic non-small cell lung cancer (NSCLC) whose tumors express the
programmed death-ligand 1 (PD-L1) surface protein.1- 2 (Fig. 1).
Pembrolizumab
extends survival and has a favorable risk-benefit profile in patients with adÂvanced,
PD-L1 positive NSCLC. It is considered a new treatment option.3
Immunomodulators have revolutionized cancer therapy, but it
should be noted that early detection and treatment of adÂverse effects is
critical. While they have a relatively mild toxicity profile, immune-mediated
adverse effects can occur and be severe.
CASE REPORT
65-year
old man with history of smoking, arterial hyperÂtension, chronic obstructive
pulmonary disease (COPD) (with a forced expiratory volume in the first second
[FEV1] of 78%), heart failure, dyslipidemia, and benign prostatic hyperplasia.
He was under the care of the Oncology Service due to a lung adenocarcinoma in
the upper right lobe. The surgical biopsy revealed the following mutation
pattern: positive for CK7, non-mutated EGFR, negative for ALK, PD-L1 positive
with a tumor proportion score (TPS) of 60%. The chest tomography showed a lung
opacity in the upper right lobe with spiculated heterogeneous borders in
pleural contact, along with nodules in the middle lobe and lower left lobe
(Fig.2).

After
five months of treatment with pembrolizumab, he developed dyspnea and cough
with a drop in oxygen saturation, so he was referred to the Pulmonology
Department. A chest X-ray was performed, revealing bilateral heterogeneous
infiltrates (Fig. 3A). The chest tomography showed an image with spiculated
borders and cavitation in the upper right lobe, extending to the lower lobe,
measuring 76 Ă— 45 mm. Additionally, there were nodules in the middle lobe and
lower left lobe with associated interstitial infiltrates. Right hilar and
mediasÂtinal lymphadenopathy were observed. A superinfection was ruled out
(Fig. 3B).

The
case was presented in a multidisciplinary medical conference, and it was agreed
to discontinue the treatment with pembrolizumab since the dyspnea and new
infiltrates were interpreted as toxicity-related. In addition, the patient
started with 40 mg/day of methylprednisolone with clear improvement after 15
days. The condition was interpreted as cryptogenic organizing pneumonia
secondary to pembroÂlizumab. After 5 months of corticosteroid treatment, the
patient no longer had respiratory symptoms and showed significant improvement
in radiological infiltrates (Fig. 4A and 4B). Treatment with steroids was
considered concluded.
During
the following months, the patient was awaiting second-line treatment. He
received radiotherapy as he deÂveloped bone metastases. Subsequently, he had
intercurrent SARS-CoV-2 infection, which required hospitalization, and he
passed away.

DISCUSSION
PD-1L
is present in tumor cells and has a protecÂtive effect on them. Pembrolizumab
is a highly selective humanized monoclonal antibody against PD-1, designed to
block the interaction between PD-1 and its ligands, thus enhancing anti-tumor
cellular activity.1
Preclinical
studies have shown that radioÂtherapy also improves anti-tumor immune reÂsponses.
In an evaluation of 98 patients comparÂing pembrolizumab treatment with and
without radiotherapy, the progression-free survival in the group receiving
pembrolizumab and radiotherapy was significantly higher than in patients
without prior radiotherapy.4 The adverse
effects of a group of over 500 patients who received pembrolizumab were ranked
in order of frequency as follows: fatigue, decreased appetite, dyspnea, and
cough. The most common immune-related adverse events were thyroid disorders
(hypothyroidism and hyÂperthyroidism). The most severe adverse events included
pleural effusion, pneumonia, dyspnea, pulmonary embolism, and pneumonitis
(occurÂring in 3.5% of patients)5.
Pneumonitis was more frequently observed in patients with a history of COPD,
asthma, or in those who had received localized chest radiotherapy. In a study
involving 915 patients treated with anti-PD-1 therapy, 43 developed
pneumonitis, and one of those patients died during the immunosuppressive
treatment. The onset of symptoms varied widely,
ranging from days to over a year. Patients presented with dyspnea and cough as
the most frequent symptoms of pneumonitis, while fever and chest pain were
reported in a smaller proportion of cases. Some patients did not show any
symptoms at the beginÂning of the pneumonitis, and over 50% showed other
immune-mediated manifestations such as hypophysitis, thyroiditis, or arthritis.
The diagÂnosis of pneumonitis is suspected
basing on the presence of new progressive pulmonary infiltrates. Computed
tomography is the imaging technique of choice. The most frequent CT findings
were cryptogenic organizing pneumonia6 and ground-glass opacities.7-8
The management of pneumonitis depends on its clinical and
radiological severity.7
On the other hand, Fujita et al reported a case of diffuse interstitial lung disease following thoracic surgery in a patient who had previously received pembrolizumab as neoadjuvant treatment. We believe that the case we presented emphasizes the importance of frequently monitoring potenÂtial adverse effects associated with the treatment of NSCLC with immunomodulators, given their potential severity and the favorable response that pulmonary toxicity caused by pembrolizumab shows to systemic corticosteroids.9
REFERENCES
1. Garon EB, Rizvi NA, Hui R, Leighl N, et al. Pembrolizumab for
the Treatment of Non–Small-Cell Lung Cancer. N Engl J Med. 2015;372:2018-28.
https://doi.org/10.1056/NEJMoa1501824
2. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA
Approval Summary: Pembrolizumab for the TreatÂment of Patients With Metastatic
Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1.
Oncologist. 2016;21:643-50. https://doi.org/10.1634/theoncologist.2015-0498
3. Leroy V, Templier C, Faivre JB, Scherpereel A, Fournier C,
Mortier L, Wemeau-Stervinou L. Pembrolizumab-induced pneumonitis. ERJ Open Res.
2017;3:00081-2016. https://doi.org/10.1183/23120541.00081-2016
4. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman
JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity
of pembrolizumab in the treatment of non-small-cell lung cancer: a secondÂary
analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895-903.
https://doi.org/10.1016/S1470-2045(17)30380-7
5. Leighl NB, Hellmann MD, Hui R, et al. Pembrolizumab in patients
with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an
open-label, phase 1 study. Lancet Respir Med. 2019;7:347-57.
https://doi.org/10.1016/S2213-2600(18)30500-9
6. Fragkou P, Souli M, Theochari M, Kontopoulou C, Loukides S,
Koumarianou A. A Case of Organizing Pneumonia (OP) Associated with
Pembrolizumab. Drug Target Insights. 2016;10:9-12.
https://doi.org/10.33393/dti.2016.1420
7. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients
Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin
Oncol. 2017;35:709-17.
https://doi.org/10.1200/JCO.2016.68.2005
8. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus
docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung
cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-50.
https://doi.org/10.1016/S0140-6736(15)01281-7
9. Fujita T, Hayama N, Kuroki T, et al. Pembrolizumab-induced
interstitial lung disease following thoracic surgery in a patient with
non-small cell lung cancer. Thorac Cancer. 2019;10:2179-82.
https://doi.org/10.1111/1759-7714.13194